Abstract
Introduction
Various strategies have been proposed for postoperative pain control. Among those, intravenous lidocaine infusion (IVLI) has gained in interest. However, its clinical benefit remains unclear. This systematic review is an evaluation of the analgesic efficacy and safety of IVLI during general anesthesia.
Methods
A systematic search was performed using MEDLINE, EMBASE, Cochrane, and SCOPUS databases, likewise, grey literature. The review included all randomized controlled trials that used a placebo or any comparator and evaluated IVLI during general anesthesia for any type of surgery. Primary outcomes were pain control and opioid requirement. Secondary outcomes were mortality, length of stay, ileus recovery time, nausea/vomiting, and adverse events. Random effects models were used and heterogeneity was assessed using the I2 index.
Results
From 5,472 citations retrieved, 29 studies involving a total of 1,754 patients met eligibility. At six hours postoperatively, intravenous lidocaine infusion reduced pain at rest (weighted mean difference [WMD] −8.70, 95% confidence intervals [CI] −16.19 to −1.21), during cough (WMD −11.19, 95% CI −17.73 to −4.65), and during movement (WMD −9.56, 95% CI −17.31 to −1.80). Intravenous lidocaine infusion also reduced opioid requirement (morphine) (WMD −8.44 mg, 95% CI −11.32 to −5.56), time to first flatus (WMD −7.62 hr, 95% CI −10.78 to −4.45), time to first feces (WMD −10.71 hr, 95% CI −16.14 to −5.28), nausea/vomiting (risk ratios = 0.71, 95% CI 0.57-0.90), and hospital length of stay (WMD −0.17 days, 95% CI −0.41 to 0.07). Abdominal surgery was strongly associated with benefit. For the 12 studies that systematically screened adverse events, the incidence of cardiac and neurologic adverse events was comparable. Eight studies observed toxic plasma levels.
Discussion
Perioperative IVLI reduced postoperative pain and opioid requirement, as well as ileus recovery time, hospital length of stay, and nausea/vomiting. Intravenous lidocaine infusion was effective mainly in abdominal surgery populations. Considering that toxic levels were detected and that adverse events were not systematically screened for in most studies, dose and safety of IVLI should be established before recommending its use.
Résumé
Introduction
Plusieurs stratégies ont été proposées pour le contrôle de la douleur postopératoire. Parmi ces stratégies, la lidocaïne par voie intraveineuse suscite un intérêt croissant. Toutefois, ses avantages cliniques demeurent peu clairs. Cette revue méthodique est une évaluation de l’efficacité analgésique et de l’innocuité de la lidocaïne par voie intraveineuse pendant l’anesthésie générale.
Méthode
Une recherche méthodique a été réalisée dans les bases de données MEDLINE, EMBASE, Cochrane et SCOPUS ainsi que dans la littérature grise. Cette revue a tenu compte de toutes les études randomisées contrôlées ayant utilisé un placebo ou un traitement de référence et évalué la lidocaïne par voie intraveineuse pendant l’anesthésie générale, quel que soit le type de chirurgie. Les principaux critères d’évaluation étaient le contrôle de la douleur et le besoin en opioïdes. Les critères d’évaluation secondaires étaient la mortalité, la durée d’hospitalisation, le temps de récupération du transit intestinal, les nausées et vomissements et les événements indésirables. Des modèles à effets aléatoires ont été utilisés et l’hétérogénéité a été évaluée à l’aide de l’indice I2.
Résultats
Des 5472 citations extraites, 29 études auxquelles un total de 1754 patients ont participé répondaient à nos critères de sélection. Six heures après l’opération, la lidocaïne par voie intraveineuse a réduit la douleur au repos (différence moyenne pondérée [DMP] −8,70, intervalles de confiance [IC] 95 % −16,19 à −1,21), pendant les épisodes de toux (DMP −11,19, IC 95 % −17,73 à −4,65), et en mouvement (DMP −9,56, IC 95 % −17,31 à −1,80). La lidocaïne par voie intraveineuse a également réduit le besoin en opioïdes (morphine) (DMP −8,44 mg, IC 95 % CI −11,32 à −5,56), le temps jusqu’à la première flatuosité (DMP −7,62 h, IC 95 % −10,78 à −4,45), le temps jusqu’à la première selle (DMP −10,71 h, IC 95 % −16,14 à −5,28), les nausées et vomissements (risques relatifs = 0,71, IC 95 % 0,57-0,90) et la durée d’hospitalisation (DMP −0,17 jours, IC 95 % −0,41 à 0,07). La chirurgie abdominale a été fortement associée aux bienfaits de la lidocaïne par voie intraveineuse. Dans les 12 études ayant méthodiquement rendu compte des événements indésirables, l’incidence d’événements indésirables cardiaques et neurologiques était comparable. Huit études ont fait état de taux plasmatiques toxiques.
Discussion
La lidocaïne par voie intraveineuse périopératoire a réduit la douleur postopératoire et le besoin d’opioïdes, ainsi que le temps de récupération du transit intestinal, la durée d’hospitalisation et les nausées et vomissements. La lidocaïne par voie intraveineuse a été particulièrement efficace chez les patients subissant une chirurgie abdominale. Étant donné que des niveaux toxiques ont été observés dans la plupart des études sans pour autant qu’une association avec les événements indésirables puisse être établie, la posologie et l’innocuité de la lidocaïne par voie intraveineuse devrait être déterminée avant de recommander son utilisation.
Similar content being viewed by others
Postoperative pain control is a major concern in anesthesia, especially in the emerging field of fast-track surgery.1,2 Anesthesiologists can choose among different interventions for postoperative pain control, but there is little evidence that different pain intervention strategies impact clinically important postoperative outcomes.3
Many years ago, intravenous infusion of lidocaine (IVLI) was proposed as an anesthesia adjuvant, but this approach has gained in interest in the medical literature only recently.4 Lidocaine has been described as having analgesic,5 anti-hyperalgesic,6 and anti-inflammatory5,7 properties. Two systematic reviews evaluating the effect of IVLI for pain relief in patients with chronic neuropathic pain or burn injury showed a potential benefit. However, its effect on postoperative outcomes is not well understood.8,9 Recently, two systematic reviews evaluated the effect of perioperative use of IVLI as an adjuvant to general anesthesia. However, one review was limited to abdominal surgery and did not include an evaluation of potential side effects of IVLI,10 while the other review included only a small number of studies due to a limited search strategy.11
Thus, we undertook a systematic review of randomized controlled trials to evaluate the efficacy and safety of perioperative IVLI on postoperative outcomes in all types of surgery requiring general anesthesia.
Methods
Search strategy
We electronically searched Ovid Medline (1950 - July 2010, Week 1), Embase (1974 - July 2010, Week 1), the Cochrane Central Register of Controlled Trials, and the Scopus database. No restrictions were used in language or type of publication. To identify relevant grey literature, we used the OpenSIGLE repository and three public search engines, i.e., Google Scholar, Intute, and the Trip databases. The search strategy was developed for Medline and adapted for each database using the same keywords. The keywords and MESH (EMTREE) terms used were divided in two main categories: 1) groups of keywords for lidocaine and 2) groups of keywords for surgery/anesthesia. We also used specific filters in Medline12 and Embase13 to limit our search to randomized control trials (RCTs). This search strategy was designed and peer-reviewed by co-investigators prior to its execution. The complete search strategy used in Medline is presented in the Appendix. We reviewed the bibliographies of all included studies to identify additional relevant publications.
Study selection
We included RCTs that evaluated the efficacy on postoperative outcomes of administering IVLI in adults (≥18 yr old) during general anesthesia. We included all comparator groups, including placebo and usual care.
Data abstraction
We developed a standardized data abstraction form. Following pilot testing of the form, data were abstracted independently by two investigators (L.V. and D.C.), and disagreements were resolved by consensus or by a third party (A.F.T.). Citations from the grey literature were adjudicated by a single reviewer (L.V.). If important data were missing or were ambiguous, corresponding authors were contacted to obtain additional information.
Outcome measures and data collected
Our primary outcomes were pain control, as assessed by visual analog scales or the equivalent, and the use of opioids. Secondary outcomes were mortality, postanesthesia care unit (PACU) and hospital length of stay, ileus recovery time (as defined by time to first flatus and/or feces), incidence of nausea or vomiting, the use of volatile anesthetics, and adverse events.
For each selected study, we collected the following population characteristics: age, sex, weight, American Society of Anesthesiologists physical status, type and length of surgery, chronic use of opioids, and factors potentially affecting lidocaine metabolism (i.e., hepatic disease and chronic use of propanolol). The study intervention characteristics collected were bolus dose, infusion rate, length of infusion, timing of infusion, total dose, and mean plasma lidocaine concentration. We also noted relevant co-interventions, such as local or regional anesthesia, including opioids or other anesthetic agents. Recorded adverse events included plasma lidocaine concentration >5 μg·mL-1 allergic reaction, neurological events (seizure, coma, tinnitus, numbness of the tongue, restlessness, vertigo, drowsiness, and apnea), or cardiovascular side effects (hypotension, arrhythmias). When data were not mutually exclusive for a specific adverse event, the highest incidence was used for analysis.
Data synthesis
Pain scores were analyzed at six, 12, 24, 48, and 72 hr following surgery. When data from one of these specific time periods were not available, data from the closest time interval were used. In one study, patients were randomized into four groups: two groups received IVLI, but with a different co-intervention, and two groups received the same comparator.14 Morphine equivalence was calculated for opioids when means ± standard deviations were reported. The following equivalents were considered: 10 mg of morphine = 100 μg of fentanyl iv,15 75 mg of meperidine im,15,16 15 mg of piritramide iv,17 or 10 μg of sufentanil iv.18
Risk of bias assessment
The methodological quality of the included studies was evaluated by two reviewers (L.V. and D.C.) using an adaptation of the scale used by the Cochrane Collaboration19,20 and the Jadad scale.21 The Cochrane Risk-of-Bias assessment tool allows a qualitative evaluation of the probability of bias (low, high, unclear) in five different potential sources of bias.
Statistical analysis
Data were analyzed with Review Manager, version 5.0 (RevMan, The Cochrane Collaboration, Oxford, United Kingdom) using random effects models. Continuous data are expressed as weighted mean difference (WMD) with 95% confidence intervals, while dichotomous data are presented as risk ratios (RR). Heterogeneity was evaluated using the I2 index.22 Sensitivity analyses were performed to identify potential sources of heterogeneity based on the following subgroups determined a priori: methodological quality (Jadad score ≥3 vs <3, placebo or other comparator, blinding), type of surgery (cardiac, abdominal, open, or laparoscopic), intervention regimen (bolus prior to infusion, infusion rate ≥3 mg·kg-1·hr-1, infusion prolonged after surgery, infusion during outcome measurement), and source of sponsorship.
We conducted the study and prepared the manuscript following the guidelines from the PRISMA statement for systematic reviews and meta-analyses (www.prisma-statement.org).
Results
Literature search
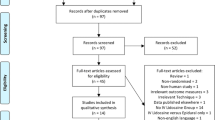
We extracted 5,072 records, excluding duplicates, from the four main databases searched (Embase, Medline, Cochrane, and Scopus) (Fig. 1), and from those we selected 32 citations for inclusion. Three studies were excluded because none of the outcomes of interest were evaluated. Searching the grey literature through OpenSIGLE and the three public search engines did not identify additional studies. Thus, 29 studies enrolling a total of 1,754 patients were ultimately included in this systematic review.
Description of included studies
All studies were written in English except one in German23 and one in Spanish.24 Seven studies originated in the USA,25-31 one in Belgium,32 two in Taiwan,14,33 three in Germany,23,34,35 one in New Zealand,36 three in Sweden,37-39 two in China,40,41 one in France,42 three in Canada,43-45 one in Mexico,24 two in Saudi Arabia,46,47 one in Israel,48 one in the Republic of Korea49 and one in Finland.50 Studies were conducted in patients undergoing abdominal, cardiac, thoracic, orthopedic, or urologic surgery (Table 1). Source of sponsorship was not mentioned in 11 studies,23-27,29,42,45-48 while other studies received grants from foundations, national councils, or universities. No study reported industry sponsorship. Descriptions of the 29 studies are summarized in Table 1.
Risk of bias assessment
Twelve studies (46%) had a low risk of bias according to the five potential sources of bias considered in the Cochrane Risk-of-Bias assessment tool (Table 2). Two studies had a high risk of bias, as they were not blinded.28,50 In contrast, 23 (79%) studies with a score ≥ 3 using the Jadad scale were considered of high methodological quality.51 Twenty-six (90%) studies were placebo-controlled blinded studies.14,23-27,29-43,45-49
Primary outcomes
Postoperative pain
Postoperative pain was evaluated in 19 studies and at different time periods, from one minute to 96 hr following surgery. Most studies presented mean pain scores,14,24,26,31-33,35,38,40,42,45,47,48 while five studies presented median pain score.23,25,34,43,44 The data from the studies reporting medians could not be included in the pooled analyses. One study27 evaluated pain with an adapted scale that could not be pooled with other data. Eighteen studies14,23-27,31-35,38,42-45,47,48 evaluated pain scores at rest, seven during cough,14,32,33,35,43,47,48 and five during movement.32,34,42,43,45 One study31 evaluated pain 24 hr after the surgery during rest and activity together and therefore was not included in our analyses.
Postoperative pain at rest
Following surgery, the WMD between groups for pain control at rest was statistically significant at six hours (nine studies, n = 579, WMD −8.70, 95% CI −16.19 to −1.21; I2 = 89%) and at 12 hr (six studies, n = 389, WMD −6.52, 95% CI −12.12 to −0.91; I2 = 79%) but not at 24, 48, or 72 hr (Fig. 2). Although a statistically significant difference was not observed between groups after 12 hr, a trend towards improved pain control at rest was observed among patients who received IVLI during the perioperative period. Sensitivity analyses showed that the benefit at 12 hr and prior and the observed trend after 12 hr may be explained by abdominal surgeries (Fig. 3).
Pain during cough
After surgery, the WMDs for pain control during cough were statistically significant among the IVLI and control groups at six hours (seven studies, n = 410, WMD −11.19, 95% CI −17.73 to −4.65; I2 = 84%), 12 hr (four studies, n = 280, WMD −7.44, 95% CI −14.24 to −0.63; I2 = 84%), and 24 hr (six studies, n = 380, WMD −6.94, 95% CI −12.87 to −1.01; I2 = 78%) favouring pain reduction for IVLI groups (Fig. 2). However, despite a clear trend in pain reduction, the WMDs were not statistically different at 48 or 72 hr. All studies were performed in abdominal surgery populations, except one40 that evaluated pain during cough at six hours after thoracic surgery. Sensitivity analyses showed consistent findings.
Pain during movement
Pain during movement after surgery was decreased significantly with IVLI at six hours (two studies, n = 130, WMD −9.56, 95% CI −17.31 to −1.80; I2 = 45%) (Fig. 2). No difference was observed at 24 or 72 hr. The only study that assessed pain during movement 12 hr after surgery observed no difference between groups.32 In abdominal surgery only, pain during movement was not significantly reduced by IVLI (Fig. 3).
Morphine equivalent administration during the postoperative period
Seventeen studies described the administration of analgesic agents during the postoperative period (from PACU to eight days following surgery). Morphine equivalent administration could be calculated for 12 of these studies.14,26,27,31,34,35,37-39,44,45,47 The mean morphine dose received in the control group was 46.4 mg. A reduction in morphine of 8.4 mg was observed in the IVLI group compared with the control group (12 studies, n = 690, WMD −8.44, 95% CI −11.32 to −5.56; I2 = 18%). This reduction was explained mainly by the type of surgery (abdominal vs non-abdominal) (Fig. 3). In the four studies presenting median opioid doses, two studies observed results consistent with these findings32,40 while two studies observed contradicting results.25,42
Secondary outcomes
Mortality
Six studies presented mortality data.26,28,30,36,41,50 Mortality in the IVLI group (0.4%) was not significantly different from that in the control group (0.5%) (six studies, n = 611, RR: 0.87, 95% CI 0.42-1.80; I2 = 0%).
Nausea or vomiting
Thirteen studies assessed the incidence of postoperative nausea or vomiting25,31-34,37,38,40,43-47 (Table 3). Pooled results showed that the incidence was significantly lower in patients who received IVLI (25%) than in those who did not (35%) (12 studies, n = 617, RR: 0.71, 95% CI 0.57-0.90; I2 = 0%).
Length of stay
The length of stay in the PACU was evaluated in only two studies.31,35 A comparable length of stay was observed in the IVLI groups and control groups in both studies.
Pooled results of hospital length of stay from nine studies showed a statistically significant reduction of about four hours for patients who received IVLI (nine studies, n = 539, WMD −0.17 day, 95% CI −0.41 to 0.07; I2 = 8%). Among studies reporting median length of stay, two studies observed a significant difference between groups in terms of hospital length of stay: two days (interquartile range [IQR] 2-3 days) and seven days (IQR 6-8 days) for the IVLI groups and three days (IQR 3-4 days) and eight days (IQR 7-11 days) for the control groups, respectively.32,35 However, in three other studies, no difference was observed between groups.25,30,44
Bowel function
Only studies conducted in abdominal surgery populations evaluated bowel function. Nine studies presented data on the time to first flatus,14,25,27,32,33,35,37,43,47 while seven studies presented data on time to first feces25,27,32,34,35,37,43 (Table 3).
Time to first flatus
Pooled analysis demonstrated a significant reduction of approximately eight hours in favour of IVLI (seven studies, n = 388, WMD −7.62 hr, 95% CI −10.78 to −4.45; I2 = 59%). All studies were conducted in cases of abdominal surgery, but statistical heterogeneity was explained by the type of abdominal surgery (open vs laparoscopic) (open surgery: four studies, n = 168, WMD −11.41 hr, 95% CI −14.36 to −8.45; I2 = 0% vs laparoscopic surgery: three studies, n = 220, WMD −5.12 hr, 95% CI −6.65 to −3.59; I2 = 0%). One additional study32 also showed a median reduction in time to first flatus in the IVLI group (17 hr, IQR 11-24 hr) compared with the control group (28 hr, IQR 25-33 hr), while another study showed no statistical difference among groups.25
Time to first feces
In the IVLI group, a statistically significant reduction of approximately 14 hr in the time to first feces was observed in the pooled analysis of studies27,35,37,43 (four studies, n = 168, WMD −10.71 hr, 95% CI −16.14 to −5.28; I2 = 0%). Data from three additional studies could not be pooled because mean times were not provided or could not be calculated.25,32,34 However, the median time to first feces in two of the studies was comparable with the results of the pooled WMD, and the third study showed no statistical differences between groups.25
Administration of volatile agents
The need for volatile agents during surgery was reported in six studies.14,29,32,33,44,47 In four of the studies, a reduction in the administration of volatile agents during surgery was observed.14,32,33,47
Adverse events
Among the 29 studies included in this systematic review, 20 reported adverse events (Table 4). Nineteen studies calculated plasma lidocaine levels.23,25-32,34-36,38,40-42,46,47,50 A plasma concentration of >5 μg·mL-1 of lidocaine was observed in eight studies,25,26,28,29,35,36,41,50 six in cardiac surgery26,28,29,36,41,50 and two in abdominal surgery.25,35 Only three studies reported the number of patients involved (three patients in total).25,26,35 There was no association between the toxic levels of lidocaine and the dose received. Among the 20 studies presenting data on adverse events, 12 studies systematically looked for cardiovascular and/or neurological adverse events.14,25,33,34,37-40,42,45-47 Overall, the incidence of adverse events between the IVLI and the control groups was comparable.
Discussion
In this study, we observed a significant difference in pain control at rest, during cough, or during movement with the use of IVLI in surgical patients under general anesthesia. This difference was associated mainly with studies conducted in abdominal surgery populations. Accordingly, we showed that opioid use was lower among patients who received IVLI. Again, this effect was observed primarily in patients undergoing abdominal surgery. Perioperative IVLI was associated with a shorter hospital length of stay, decreased incidence of nausea or vomiting, and faster return of bowel function, with no impact on hospital mortality. The incidence of adverse events was not well-documented, but a few studies reported critical levels of lidocaine, an observation that requires further investigation.
Comparison with previous studies
The significant difference in the quality of pain control observed in studies involving patients who underwent abdominal surgery was also observed in two recent meta-analyses performed in this patient subgroup.10,11 However, one of these studies was limited to a specific surgical population and a specific period of evaluation (24 hr) following surgery.10 On the contrary, our study evaluated the quality of pain control at five different time periods over the first 72 hr following surgery. In addition, our study was more exhaustive and methodologically rigorous and used a thorough search strategy, which led to the screening of more than 5,472 citations to include 29 studies and more than 1,754 patients. In comparison, only eight studies (n = 360) and 16 studies (n = 764) were identified for inclusion in the previous meta-analyses, respectively.10,11 In addition, studies conducted in children were included in one of these systematic reviews, a population where the biodisponibility and effect of the drug could be different.11 Moreover, the standard error of mean, which several studies used as a measure of dispersion of the different outcomes, was interpreted as a standard deviation in these two meta-analyses. This interpretation would have wrongly increased the precision of the outcome measure of the included studies and, thus, the point estimate of the pooled analysis. In our study, we calculated the appropriate standard deviations before conducting the pooled analyses. This approach allowed a broader description of the effect of IVLI on pain control over a clinically significant period. Moreover, rather than focusing on only a specific subgroup of patients, we conducted a series of sensitivity analyses, including stratification by the type of surgery, allowing a more exhaustive comprehension of the potential effect of IVLI. We also conducted a systematic evaluation of potential side effects of IVLI during the perioperative setting.
It remains unknown why patients undergoing abdominal surgery may particularly benefit from IVLI. One explanation might be differences in pain mechanisms; visceral pain from abdominal surgery might be triggered by mechanisms other than non-visceral pain, such as bone and cartilage trauma in orthopedic or cardiac surgeries. Part of the discomfort and pain following abdominal surgery might also be related to postoperative ileus and nausea/vomiting. Indeed, the reduction observed in the time to bowel function recovery as well as the decreased incidence of nausea/vomiting might explain why the improvement in pain control was observed mainly in studies based on abdominal surgery.
Limitations and strength
The main limitation of our study is related to the methodological quality of the included RCTs. First, our findings were based on small-sized single-centre RCTs. Despite the use of two different instruments to assess the potential risk of bias and methodological quality of included studies,19,21 RCTs that are considered to have a low risk of bias or to be of high-methodological quality could have some important design flaws not detected by methodological evaluations. For example, some studies considered to be double-blinded did not mention if data collectors were blinded to the treatment regimen.26,29,36-39,41 Second, pooled analyses were limited by data availability in studies meeting inclusion criteria (i.e., fewer studies evaluated pain during cough or movement as opposed to at rest). Therefore, a type II error cannot be excluded in certain analyses. In addition, only median values were presented in several studies and, therefore, could not be included in the pooled analyses since the mean values could not be estimated.23,25,32,34,35,42,44 Furthermore, not all planned sensitivity analyses could be performed due to insufficient available data, despite efforts made to obtain these data by contacting the corresponding authors of the studies. Thus, the observed statistical heterogeneity in certain analyses could not always be explained. Moreover, despite the fact that sensitivity analyses did not reveal a statistically significant influence as a result of dose regimen and duration of therapy, we cannot exclude the possibility of a clinically important impact on the efficacy and safety of IVLI. Indeed, the included studies made no mention of having conducted a dose-response study prior to establishing their protocol, and they made no reference regarding the need to establish the ideal dose prior to conducting the RCT. The use of non-optimal dosage regimens of IVLI in some of the included studies might have had an impact on the results observed and, thus, on the results of our meta-analysis.
The main strength of our study stands in its design aimed at presenting an exhaustive evaluation of the effect of IVLI use during the perioperative period. First, the search was systematic, including grey literature and a manual search of the references of included studies and conference proceedings. Moreover, by targeting a broad population of surgical patients, we were able to provide a clear description of both the potentially beneficial and harmful effects of this analgesic modality. In addition, sensitivity analyses determined a priori enabled us to assess sources of heterogeneity when present, and also to identify the subpopulation of patients that could potentially benefit from the therapy. Our study provides an accurate and comprehensive understanding of the effects of IVLI on perioperative outcomes, including safety issues.
In summary, IVLI as an anesthesia adjuvant during general anesthesia has the potential to improve postoperative analgesia and the efficiency of care, mainly in the context of abdominal surgeries. However, the safety of IVLI cannot be confirmed considering its association with potentially serious adverse events and the scarcity of studies that have systematically assessed the incidence of adverse events, including toxic plasmatic levels of lidocaine. Thus, before its use can be recommended, further research is required, especially where it pertains to an optimal dosage regimen and the safety of this intervention.
References
White PF, Kehlet H, Neal JM, et al. The role of the anesthesiologist in fast-track surgery: from multimodal analgesia to perioperative medical care. Anesth Analg 2007; 104: 1380-96.
Roediger L, Larbuisson R, Lamy M. New approaches and old controversies to postoperative pain control following cardiac surgery. Eur J Anaesthesiol 2006; 23: 539-50.
Liu SS, Wu CL. Effect of postoperative analgesia on major postoperative complications: a systematic update of the evidence. Anesth Analg 2007; 104: 689-702.
Omote K. Intravenous lidocaine to treat postoperative pain management: novel strategy with a long-established drug. Anesthesiology 2007; 106: 5-6.
Lauretti GR. Mechanisms of analgesia of intravenous lidocaine. Revista Bras Anestesiol 2008; 58: 280-6.
Koppert W, Ostermeier N, Sittl R, Weidner C, Schmelz M. Low-dose lidocaine reduces secondary hyperalgesia by a central mode of action. Pain 2000; 85: 217-24.
Hollmann MW, Durieux ME. Local anesthetics and the inflammatory response: a new therapeutic indication? Anesthesiology 2000; 93: 858-75.
Challapalli V, Tremont-Lukats IW, McNicol ED, Lau J, Carr DB. Systemic administration of local anesthetic agents to relieve neuropathic pain. Cochrane Database Syst Rev 2005; 4: CD003345.
Wasiak J, Cleland H. Lidocaine for pain relief in burn injured patients. Cochrane Database Syst Rev 2007; 3: CD005622.
Marret E, Rolin M, Beaussier M, Bonnet F. Meta-analysis of intravenous lidocaine and postoperative recovery after abdominal surgery. Br J Surg 2008; 95: 1331-8.
McCarthy GC, Megalla SA, Habib AS. Impact of intravenous lidocaine infusion on postoperative analgesia and recovery from surgery: a systematic review of randomized controlled trials. Drugs 2010; 70: 1149-63.
Glanville JM, Lefebvre C, Miles JN, Camosso-Stefinovic J. How to identify randomized controlled trials in MEDLINE: ten years on. J Med Libr Assoc 2006; 94: 130-6.
Wong SS, Wilczynski NL, Haynes RB. Developing optimal search strategies for detecting clinically sound treatment studies in EMBASE. J Med Libr Assoc 2006; 94: 41-7.
Wu CT, Borel CO, Lee MS, et al. The interaction effect of perioperative cotreatment with dextromethorphan and intravenous lidocaine on pain relief and recovery of bowel function after laparoscopic cholecystectomy. Anesth Analg 2005; 100: 448-53.
Gammaitoni AR, Fine P, Alvarez N, McPherson ML, Bergmark S. Clinical application of opioid equianalgesic data. Clin J Pain 2003; 19: 286-97.
Berry PH, Chapman CR, Covington EC, et al. Pain: current understanding of assessment, management, and treatments. In: Council NP, editor. 2001.
Hudcova J, McNicol E, Quah C, Lau J, Carr DB. Patient controlled opioid analgesia versus conventional opioid analgesia for postoperative pain. Cochrane Database Syst Rev 2006; 4: CD003348.
Van De Walle J, Lauwers P, Adriaensen H. Double blind comparison of fentanyl and sulfentanil in anesthesia. Acta Anaesthesiol Belg 1976; 27: 129-38.
Deshauer D, Moher D, Fergusson D, Moher E, Sampson M, Grimshaw J. Selective serotonin reuptake inhibitors for unipolar depression: a systematic review of classic long-term randomized controlled trials. CMAJ 2008; 178: 1293-301.
Higgins JP, Altman DG (editors), On behalf of the Cochrane Statistical Methods Group and the Cochrane Bias Methods Group. Chapter 8: Assessing risk of bias in included studies. In: Higgins JP, Green S editors. Cochrane Handbook for Systematic Reviews of Interventions Version 5.0.1 (updated September 2008). The Cochrane Collaboration, 2008. Available from www.cochrane-handbook.org. Accessed September 2010.
Jadad AR, Moore RA, Carroll D, et al. Assessing the quality of reports of randomized clinical trials: is blinding necessary? Control Clin Trials 1996; 17: 1-12.
Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ 2003; 327: 557-60.
Striebel HW, Klettke U. Is intravenous lidocaine infusion suitable for postoperative pain management? Schmerz 1992; 6: 245-50 (German).
Juarez-Pichardo JS, Avila-Lopez A, Serrano-Herrera MA. Preventive postoperative analgesia with dexmetomidine iv compared to lidocaine iv in cholecystectomy. Revista Mex Anestesiol 2009; 32: 81-8.
Swenson BR, Gottschalk A, Wells LT, et al. Intravenous lidocaine is as effective as epidural bupivacaine in reducing ileus duration, hospital stay, and pain after open colon resection: a randomized clinical trial. Reg Anesth Pain Med 2010; 35: 370-6.
Insler SR, O’Connor M, Samonte AF, Bazaral MG. Lidocaine and the inhibition of postoperative pain in coronary artery bypass patients. J Cardiothorac Vasc Anesth 1995; 9: 541-6.
Groudine SB, Fisher HA, Kaufman RP Jr, et al. Intravenous lidocaine speeds the return of bowel function, decreases postoperative pain, and shortens hospital stay in patients undergoing radical retropubic prostatectomy. Anesth Analg 1998; 86: 235-9.
Knight PR, Kroll DA, Nahrwold ML, et al. Comparison of cardiovascular responses to anesthesia and operation when intravenous lidocaine or morphine sulfate is used as adjunct to diazepam-nitrous oxide anesthesia for cardiac surgery. Anesth Analg 1980; 59: 130-9.
Kasten GW, Owens E. Evaluation of lidocaine as an adjunct to fentanyl anesthesia for coronary artery bypass graft surgery. Anesth Analg 1986; 65: 511-5.
Mathew JP, Mackensen GB, Phillips-Bute B, et al. Randomized, double-blinded, placebo controlled study of neuroprotection with lidocaine in cardiac surgery. Stroke 2009; 40: 880-7.
McKay A, Gottschalk A, Ploppa A, Durieux ME, Groves DS. Systemic lidocaine decreased the perioperative opioid analgesic requirements but failed to reduce discharge time after ambulatory surgery. Anesth Analg 2009; 109: 1805-8.
Kaba A, Laurent SR, Detroz BJ, et al. Intravenous lidocaine infusion facilitates acute rehabilitation after laparoscopic colectomy. Anesthesiology 2007; 106: 11-8; discussion 5-6.
Kuo CP, Jao SW, Chen KM, et al. Comparison of the effects of thoracic epidural analgesia and i.v. infusion with lidocaine on cytokine response, postoperative pain and bowel function in patients undergoing colonic surgery. Br J Anaesth 2006; 97: 640-6.
Koppert W, Weigand M, Neumann F, et al. Perioperative intravenous lidocaine has preventive effects on postoperative pain and morphine consumption after major abdominal surgery. Anesth Analg 2004; 98: 1050-5.
Herroeder S, Pecher S, Schonherr ME, et al. Systemic lidocaine shortens length of hospital stay after colorectal surgery: a double-blinded, randomized, placebo-controlled trial. Ann Surg 2007; 246: 192-200.
Mitchell SJ, Pellett O, Gorman DF. Cerebral protection by lidocaine during cardiac operations. Ann Thorac Surg 1999; 67: 1117-24.
Rimback G, Cassuto J, Tollesson PO. Treatment of postoperative paralytic ileus by intravenous lidocaine infusion. Anesth Analg 1990; 70: 414-9.
Cassuto J, Wallin G, Hogstrom S, Faxen A, Rimback G. Inhibition of postoperative pain by continuous low-dose intravenous infusion of lidocaine. Anesth Analg 1985; 64: 971-4.
Wallin G, Cassuto J, Hogstrom S, et al. Effects of lidocaine infusion on the sympathetic response to abdominal surgery. Anesth Analg 1987; 66: 1008-13.
Cui W, Li Y, Li S, Wang R, Li J. Systemic administration of lidocaine reduces morphine requirements and postoperative pain of patients undergoing thoracic surgery after propofol-remifentanil-based anaesthesia. Eur J Anaesthesiol 2010; 27: 41-6.
Wang D, Wu X, Li J, Xiao F, Liu X, Meng M. The effect of lidocaine on early postoperative cognitive dysfunction after coronary artery bypass surgery. Anesth Analg 2002; 95: 1134-41.
Martin F, Cherif K, Gentili ME, et al. Lack of impact of intravenous lidocaine on analgesia, functional recovery, and nociceptive pain threshold after total hip arthroplasty. Anesthesiology 2008; 109: 118-23.
Lauwick S, Kim DJ, Mistraletti G, Carli F. Functional walking capacity as an outcome measure of laparoscopic prostatectomy: the effect of lidocaine infusion. Br J Anaesth 2009; 103: 213-9.
Lauwick S, Kim do J, Michelagnoli G, et al. Intraoperative infusion of lidocaine reduces postoperative fentanyl requirements in patients undergoing laparoscopic cholecystectomy. Can J Anesth 2008; 55: 754-60.
Bryson GL, Charapov I, Krolczyk G, Taljaard M, Reid D. Intravenous lidocaine does not reduce length of hospital stay following abdominal hysterectomy. Can J Anesth 2010; 57: 759-66.
El-Tahan MR, Warda OM, Diab DG, Ramzy EA, Matter MK. A randomized study of the effects of perioperative i.v. lidocaine on hemodynamic and hormonal responses for cesarean section. J Anesth 2009; 23: 215-21.
Saadawy IM, Kaki AM, Abd El Latif AA, Abd-Elmaksoud AM, Tolba OM. Lidocaine vs. magnesium: effect on analgesia after a laparoscopic cholecystectomy. Acta Anaesthesiol Scand 2010; 54: 549-56.
Yardeni IZ, Beilin B, Mayburd E, Levinson Y, Bessler H. The effect of perioperative intravenous lidocaine on postoperative pain and immune function. Anesth Analg 2009; 109: 1464-9.
Kim WJ, Sim JY, Choi IC. Impact of intravenous lidocaine on myocardial injury during off pump coronary artery surgery. J Cardiothorac Vasc Anesth 2010; 24: S63-4.
Rinne T, Kaukinen S. Does lidocaine protect the heart during coronary revascularisation? Acta Anaesthesiol Scand 1998; 42: 936-40.
Higgins JP, Green S. In: Green S, Higgins JP, editors. Cochrane Handbook for Systematic Reviews of Interventions. Oxford: The Cochrane Collaboration; 2008.
Acknowledgements
We sincerely thank all authors who provided additional particulars to their published data, especially Drs Ilya Charapov and Gregory Bryson. The authors also thank Mrs. Lucie Côté for her help in retrieving the identified citations.
Financial support
Drs Turgeon and Lauzier are recipients of a career research award from the Fonds de la Recherche en Santé du Québec (FRSQ). Dr. Moore is a recipient of a post-doc fellowship grant from the Canadian Institutes for Health Research (CIHR). Drs Fergusson and McIntyre are recipients of a New Investigator Award from the CIHR.
Conflict of interest
None declared.
Author information
Authors and Affiliations
Corresponding author
Appendix
Appendix
Search strategy for Medline
-
1.
Clinical trial:.pt. (462496)
-
2.
Animals/(4297276)
-
3.
Humans/(10548308)
-
4.
2 not (2 and 3) (3233613)
-
5.
1 not 4 (457386)
-
6.
Lidocaine/or Anesthetics, Local/or Local anesthetic*.mp. (36784)
-
7.
lidocaine.mp. (22522)
-
8.
xylocaine.mp. (856)
-
9.
6 or 7 or 8 (39995)
-
10.
Anesthes*.mp. [mp = title, original title, abstract, name of substance word, subject heading word] (182287)
-
11.
Anesthesia, Conduction/or Anesthesiology/or Anesthesia, General/(46074)
-
12.
Intraoperative*.mp. or Intraoperative Care/or Intraoperative Period/(85683)
-
13.
Perioperative Care/or Perioperative*.mp. (38536)
-
14.
Postoperative*.mp. or Postoperative Care/or Pain, Postoperative/or Postoperative Period/(465147)
-
15.
Surgical Procedures, Operative/or Operative Procedure*.mp. (53134)
-
16.
Surgical Procedure*.mp. (191032)
-
17.
Surger*.mp. (575864)
-
18.
Surgery/(27904)
-
19.
10 or 11 or 12 or 13 or 14 or 15 or 16 or 17 or 18 (1131018)
-
20.
5 and 9 and 19 (4374)
-
21.
local anesthesia.mp. or Anesthesia, Local/(17264)
-
22.
20 not 21 (3313)
-
23.
Anesthesia, Spinal/or regional anesth*.mp. (11040)
-
24.
22 not 23 (2637)
-
25.
Peripheral Nerves/or Nerve Block/or peripheral nerve block.mp. (28815)
-
26.
24 not 25 (2116)
-
27.
limit 26 to (humans and “all adult (19 plus years)”) (1621)
Rights and permissions
About this article
Cite this article
Vigneault, L., Turgeon, A.F., Côté, D. et al. Perioperative intravenous lidocaine infusion for postoperative pain control: a meta-analysis of randomized controlled trials. Can J Anesth/J Can Anesth 58, 22–37 (2011). https://doi.org/10.1007/s12630-010-9407-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12630-010-9407-0