Abstract
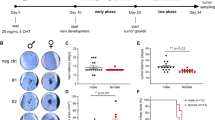
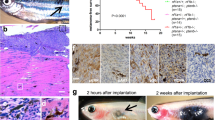
Mutational activation of BRAF is the earliest and most common genetic alteration in human melanoma. To build a model of human melanoma, we generated mice with conditional melanocyte-specific expression of BRafV600E. Upon induction of BRafV600E expression, mice developed benign melanocytic hyperplasias that failed to progress to melanoma over 15–20 months. By contrast, expression of BRafV600E combined with Pten tumor suppressor gene silencing elicited development of melanoma with 100% penetrance, short latency and with metastases observed in lymph nodes and lungs. Melanoma was prevented by inhibitors of mTorc1 (rapamycin) or MEK1/2 (PD325901) but, upon cessation of drug administration, mice developed melanoma, indicating the presence of long-lived melanoma-initiating cells in this system. Notably, combined treatment with rapamycin and PD325901 led to shrinkage of established melanomas. These mice, engineered with a common genetic profile to human melanoma, provide a system to study melanoma's cardinal feature of metastasis and for preclinical evaluation of agents designed to prevent or treat metastatic disease.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 12 print issues and online access
$209.00 per year
only $17.42 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout







Similar content being viewed by others
References
Chin, L., Merlino, G. & DePinho, R.A. Malignant melanoma: modern black plague and genetic black box. Genes Dev. 12, 3467–3481 (1998).
Gray-Schopfer, V.C., da Rocha Dias, S. & Marais, R. The role of B-RAF in melanoma. Cancer Metastasis Rev. 24, 165–183 (2005).
Chin, L. The genetics of malignant melanoma: lessons from mouse and man. Nat. Rev. Cancer 3, 559–570 (2003).
Davies, H. et al. Mutations of the BRAF gene in human cancer. Nature 417, 949–954 (2002).
Garraway, L.A. et al. Integrative genomic analyses identify MITF as a lineage survival oncogene amplified in malignant melanoma. Nature 436, 117–122 (2005).
Hayward, N.K. Genetics of melanoma predisposition. Oncogene 22, 3053–3062 (2003).
Pollock, P.M. et al. High frequency of BRAF mutations in nevi. Nat. Genet. 33, 19–20 (2003).
Wellbrock, C., Karasarides, M. & Marais, R. The RAF proteins take centre stage. Nat. Rev. Mol. Cell Biol. 5, 875–885 (2004).
Mercer, K.E. & Pritchard, C.A. Raf proteins and cancer: B-Raf is identified as a mutational target. Biochim. Biophys. Acta 1653, 25–40 (2003).
Michaloglou, C. et al. BRAFE600-associated senescence-like cell cycle arrest of human naevi. Nature 436, 720–724 (2005).
Sviderskaya, E.V. et al. p16(Ink4a) in melanocyte senescence and differentiation. J. Natl. Cancer Inst. 94, 446–454 (2002).
Wellbrock, C. et al. V599EB-RAF is an oncogene in melanocytes. Cancer Res. 64, 2338–2342 (2004).
Chin, L., Garraway, L.A. & Fisher, D.E. Malignant melanoma: genetics and therapeutics in the genomic era. Genes Dev. 20, 2149–2182 (2006).
Lin, W.M. et al. Modeling genomic diversity and tumor dependency in malignant melanoma. Cancer Res. 68, 664–673 (2008).
Tsao, H., Goel, V., Wu, H., Yang, G. & Haluska, F.G. Genetic interaction between NRAS and BRAF mutations and PTEN/MMAC1 inactivation in melanoma. J. Invest. Dermatol. 122, 337–341 (2004).
Tsao, H., Zhang, X., Fowlkes, K. & Haluska, F.G. Relative reciprocity of NRAS and PTEN/MMAC1 alterations in cutaneous melanoma cell lines. Cancer Res. 60, 1800–1804 (2000).
Dankort, D. et al. A new mouse model to explore the initiation, progression, and therapy of BRAFV600E-induced lung tumors. Genes Dev. 21, 379–384 (2007).
Tonks, I.D. et al. Tyrosinase-Cre mice for tissue-specific gene ablation in neural crest and neuroepithelial-derived tissues. Genesis 37, 131–138 (2003).
Guyonneau, L., Murisier, F., Rossier, A., Moulin, A. & Beermann, F. Melanocytes and pigmentation are affected in dopachrome tautomerase knockout mice. Mol. Cell. Biol. 24, 3396–3403 (2004).
Bosenberg, M. et al. Characterization of melanocyte-specific inducible Cre recombinase transgenic mice. Genesis 44, 262–267 (2006).
Bennett, D.C. Human melanocyte senescence and melanoma susceptibility genes. Oncogene 22, 3063–3069 (2003).
Jonsson, G. et al. Genomic profiling of malignant melanoma using tiling-resolution arrayCGH. Oncogene 26, 4738–4748 (2007).
Willmore-Payne, C., Holden, J.A., Hirschowitz, S. & Layfield, L.J. BRAF and c-kit gene copy number in mutation-positive malignant melanoma. Hum. Pathol. 37, 520–527 (2006).
Trotman, L.C. et al. Pten dose dictates cancer progression in the prostate. PLoS Biol. 1, E59 (2003).
Inoue-Narita, T. et al. Pten deficiency in melanocytes results in resistance to hair graying and susceptibility to carcinogen-induced melanomagenesis. Cancer Res. 68, 5760–5768 (2008).
Jimenez, M., Tsukamoto, K. & Hearing, V.J. Tyrosinases from two different loci are expressed by normal and by transformed melanocytes. J. Biol. Chem. 266, 1147–1156 (1991).
Hodi, F.S. et al. Major response to imatinib mesylate in KIT-mutated melanoma. J. Clin. Oncol. 26, 2046–2051 (2008).
Ohren, J.F. et al. Structures of human MAP kinase kinase 1 (MEK1) and MEK2 describe novel noncompetitive kinase inhibition. Nat. Struct. Mol. Biol. 11, 1192–1197 (2004).
Sabatini, D.M., Erdjument-Bromage, H., Lui, M., Tempst, P. & Snyder, S.H. RAFT1: a mammalian protein that binds to FKBP12 in a rapamycin-dependent fashion and is homologous to yeast TORs. Cell 78, 35–43 (1994).
Sheridan, C., Brumatti, G. & Martin, S.J. Oncogenic B-RafV600E inhibits apoptosis and promotes ERK-dependent inactivation of Bad and Bim. J. Biol. Chem. 283, 22128–22135 (2008).
Cartlidge, R.A. et al. Oncogenic BRAF(V600E) inhibits BIM expression to promote melanoma cell survival. Pigment Cell Melanoma Res. 21, 534–544 (2008).
Walker, G.J. & Hayward, N.K. Pathways to melanoma development: lessons from the mouse. J. Invest. Dermatol. 119, 783–792 (2002).
Bardeesy, N., Wong, K.K., DePinho, R.A. & Chin, L. Animal models of melanoma: recent advances and future prospects. Adv. Cancer Res. 79, 123–156 (2000).
Tietze, M.K. & Chin, L. Murine models of malignant melanoma. Mol. Med. Today 6, 408–410 (2000).
Woods, D. et al. Raf-induced proliferation or cell cycle arrest is determined by the level of Raf activity with arrest mediated by p21Cip1. Mol. Cell. Biol. 17, 5598–5611 (1997).
Zhu, J., Woods, D., McMahon, M. & Bishop, J.M. Senescence of human fibroblasts induced by oncogenic Raf. Genes Dev. 12, 2997–3007 (1998).
Olive, K.P. & Tuveson, D.A. The use of targeted mouse models for preclinical testing of novel cancer therapeutics. Clin. Cancer Res. 12, 5277–5287 (2006).
Patton, E.E. et al. BRAF mutations are sufficient to promote nevi formation and cooperate with p53 in the genesis of melanoma. Curr. Biol. 15, 249–254 (2005).
Chudnovsky, Y., Adams, A.E., Robbins, P.B., Lin, Q. & Khavari, P.A. Use of human tissue to assess the oncogenic activity of melanoma-associated mutations. Nat. Genet. 37, 745–749 (2005).
Freeman, D.J. et al. PTEN tumor suppressor regulates p53 protein levels and activity through phosphatase-dependent and -independent mechanisms. Cancer Cell 3, 117–130 (2003).
Larue, L. & Delmas, V. The WNT/Beta-catenin pathway in melanoma. Front. Biosci. 11, 733–742 (2006).
Landi, M.T. et al. MC1R germline variants confer risk for BRAF-mutant melanoma. Science 313, 521–522 (2006).
Eisen, T. et al. Sorafenib in advanced melanoma: a Phase II randomised discontinuation trial analysis. Br. J. Cancer 95, 581–586 (2006).
Flaherty, K.T. Chemotherapy and targeted therapy combinations in advanced melanoma. Clin. Cancer Res. 12, 2366s–2370s (2006).
Lutzky, J., Bauer, J. & Bastian, B.C. Dose-dependent, complete response to imatinib of a metastatic mucosal melanoma with a K642E KIT mutation. Pigment Cell Melanoma Res 21, 492–493 (2008).
Maira, S.M. et al. Identification and characterization of NVP-BEZ235, a new orally available dual phosphatidylinositol 3-kinase/mammalian target of rapamycin inhibitor with potent in vivo antitumor activity. Mol. Cancer Ther. 7, 1851–1863 (2008).
Tsai, J. et al. Discovery of a selective inhibitor of oncogenic B-Raf kinase with potent antimelanoma activity. Proc. Natl. Acad. Sci. USA 105, 3041–3046 (2008).
Yeh, T.C. et al. Biological characterization of ARRY-142886 (AZD6244), a potent, highly selective mitogen-activated protein kinase kinase 1/2 inhibitor. Clin. Cancer Res. 13, 1576–1583 (2007).
Sosman, J.A. & Puzanov, I. Molecular targets in melanoma from angiogenesis to apoptosis. Clin. Cancer Res. 12, 2376s–2383s (2006).
Rodriguez, C.I. et al. High-efficiency deleter mice show that FLPe is an alternative to Cre-loxP. Nat. Genet. 25, 139–140 (2000).
Acknowledgements
We thank the members of the McMahon and Bosenberg laboratories as well as B. Bastian, L. Chin, E. Filenova, B. Hann, M. Herlyn, L. Johnson, G. Merlino, P. P. Pandolfi, V. Hearing, M. Held, G. Kay and D. Matzen for the provision of mouse strains, reagents, advice and support. M.M. thanks A. Ricart and J. Sebolt-Leopold (Pfizer) for provision of PD325901 and acknowledges the support of the University of California San Francisco Helen Diller Family Comprehensive Cancer Center Mouse Pathology and Pre-Clinical Therapeutics cores. R.A.D. is supported as an American Cancer Society Research Professor. This work was supported by grants from the Melanoma Research Foundation, U.C. Discovery Award and from the US National Institutes of Health (CA 108972 to M.M., CA 84313 to R.A.D. and CA 89124 and CA 112054 to M.B., respectively).
Author information
Authors and Affiliations
Contributions
M.M. and M.B. established a collaboration for the exchange of relevant mouse strains to enable the generation and analysis of Tyr::CreER, BrafCA, Ptenlox mice and contributed to the manuscript equally as senior authors. D.D. (UCSF) and D.P.C. (University of Vermont) performed all of the mouse experiments in parallel. Representative figures were selected for publication by D.D., D.P.C., M.M. and M.B. and were prepared for publication by D.D. R.A.C. performed immunoblot analysis of 2697T cells treated with PD325901. B.N. and W.E.D. assisted with and optimized mouse tumor induction and performed immunohistochemical analysis. A.N.K. analyzed Tyr::CreER, BrafCA, Ptenlox4-5 mice treated with multiple cycles of PD325901. M.J.Y. generated and characterized Ptenlox5 mice in the laboratory of R.A.D. M.M. wrote the manuscript and shepherded it through review with contributions from D.D., D.P.C., M.J.Y., R.A.D. and M.B.
Corresponding authors
Supplementary information
Supplementary Text and Figures
Supplementary Figures 1–6, Tables 1 and 2 (PDF 2651 kb)
Rights and permissions
About this article
Cite this article
Dankort, D., Curley, D., Cartlidge, R. et al. BrafV600E cooperates with Pten loss to induce metastatic melanoma. Nat Genet 41, 544–552 (2009). https://doi.org/10.1038/ng.356
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/ng.356
This article is cited by
-
Cooperative function of oncogenic MAPK signaling and the loss of Pten for melanoma migration through the formation of lamellipodia
Scientific Reports (2024)
-
PTEN-restoration abrogates brain colonisation and perivascular niche invasion by melanoma cells
British Journal of Cancer (2024)
-
Logic-based modeling and drug repurposing for the prediction of novel therapeutic targets and combination regimens against E2F1-driven melanoma progression
BMC Chemistry (2023)
-
Landscape of enhancer disruption and functional screen in melanoma cells
Genome Biology (2023)
-
CXCR2 expression during melanoma tumorigenesis controls transcriptional programs that facilitate tumor growth
Molecular Cancer (2023)