Abstract
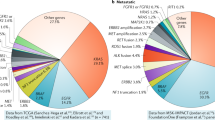
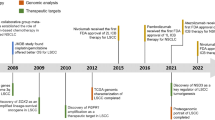
Squamous-cell lung cancer is one of the most prevalent subtypes of lung cancer worldwide and its pathogenesis is closely linked with tobacco exposure. Unfortunately, squamous-cell lung cancer patients do not benefit from major advances in the development of targeted therapeutics such as epidermal growth factor receptor (EGFR) inhibitors or anaplastic lymphoma kinase (ALK) inhibitors that show exquisite activity in lung adenocarcinomas with EGFR mutations or echinoderm microtubule associated protein like-4 (EML4)-ALK fusions, respectively. Major efforts have been launched to characterize the genomes of squamous-cell lung cancers. Among the new results emanating from these efforts are amplifications of the fibroblast growth factor receptor 1 gene and mutations of the discoidin domain receptor 2 gene as potential novel targets for the treatment of squamous-cell lung cancer patients. Here, we provide a review on these discoveries and their implications for clinical trials in squamous-cell lung cancer assessing the value of novel therapeutics addressing these targets.
This is a preview of subscription content, access via your institution
Access options
Subscribe to this journal
Receive 50 print issues and online access
$259.00 per year
only $5.18 per issue
Buy this article
- Purchase on Springer Link
- Instant access to full article PDF
Prices may be subject to local taxes which are calculated during checkout


Similar content being viewed by others
References
Thomas RK, Baker AC, Debiasi RM, Winckler W, Laframboise T, Lin WM et al. High-throughput oncogene mutation profiling in human cancer. Nat Genet 2007; 39: 347–351.
Thomas RK, Nickerson E, Simons JF, Janne PA, Tengs T, Yuza Y et al. Sensitive mutation detection in heterogeneous cancer specimens by massively parallel picoliter reactor sequencing. Nat Med 2006; 12: 852–855.
Shendure J, Porreca GJ, Reppas NB, Lin X, McCutcheon JP, Rosenbaum AM et al. Accurate multiplex polony sequencing of an evolved bacterial genome. Science 2005; 309: 1728–1732.
Harris TD, Buzby PR, Babcock H, Beer E, Bowers J, Braslavsky I et al. Single-molecule DNA sequencing of a viral genome. Science 2008; 320: 106–109.
Lipson D, Raz T, Kieu A, Jones DR, Giladi E, Thayer E et al. Quantification of the yeast transcriptome by single-molecule sequencing. Nat Biotechnol 2009; 27: 652–658.
TCGA. Comprehensive genomic characterization defines human glioblastoma genes and core pathways. Nature 2008; 455: 1061–1068.
Pao W, Miller V, Zakowski M, Doherty J, Politi K, Sarkaria I et al. EGF receptor gene mutations are common in lung cancers from ‘never smokers’ and are associated with sensitivity of tumors to gefitinib and erlotinib. Proc Natl Acad Sci USA 2004; 101: 13306–13311.
Paez JG, Janne PA, Lee JC, Tracy S, Greulich H, Gabriel S et al. EGFR mutations in lung cancer: correlation with clinical response to gefitinib therapy. Science 2004; 304: 1497–1500.
Lynch TJ, Bell DW, Sordella R, Gurubhagavatula S, Okimoto RA, Brannigan BW et al. Activating mutations in the epidermal growth factor receptor underlying responsiveness of non-small-cell lung cancer to gefitinib. N Engl J Med 2004; 350: 2129–2139.
Rosell R, Moran T, Queralt C, Porta R, Cardenal F, Camps C et al. Screening for epidermal growth factor receptor mutations in lung cancer. N Engl J Med 2009; 361: 958–967.
Mok TS, Wu YL, Thongprasert S, Yang CH, Chu DT, Saijo N et al. Gefitinib or carboplatin-paclitaxel in pulmonary adenocarcinoma. N Engl J Med 2009; 361: 947–957.
Maemondo M, Inoue A, Kobayashi K, Sugawara S, Oizumi S, Isobe H et al. Gefitinib or chemotherapy for non-small-cell lung cancer with mutated EGFR. N Engl J Med 2010; 362: 2380–2388.
Soda M, Choi YL, Enomoto M, Takada S, Yamashita Y, Ishikawa S et al. Identification of the transforming EML4-ALK fusion gene in non-small-cell lung cancer. Nature 2007; 448: 561–566.
Kwak EL, Bang YJ, Camidge DR, Shaw AT, Solomon B, Maki RG et al. Anaplastic lymphoma kinase inhibition in non-small-cell lung cancer. N Engl J Med 2010; 363: 1693–1703.
Sun Y, Ren Y, Fang Z, Li C, Fang R, Gao B et al. Lung adenocarcinoma from East Asian never-smokers is a disease largely defined by targetable oncogenic mutant kinases. J Clin Oncol 2010; 28: 4616–4620.
Pirker R, Pereira JR, Szczesna A, von Pawel J, Krzakowski M, Ramlau R et al. Cetuximab plus chemotherapy in patients with advanced non-small-cell lung cancer (FLEX): an open-label randomised phase III trial. Lancet 2009; 373: 1525–1531.
Sandler A, Gray R, Perry MC, Brahmer J, Schiller JH, Dowlati A et al. Paclitaxel-carboplatin alone or with bevacizumab for non-small-cell lung cancer. N Engl J Med 2006; 355: 2542–2550.
Scagliotti G, Novello S, von Pawel J, Reck M, Pereira JR, Thomas M et al. Phase III study of carboplatin and paclitaxel alone or with sorafenib in advanced non-small-cell lung cancer. J Clin Oncol 2010; 28: 1835–1842.
Bass AJ, Watanabe H, Mermel CH, Yu S, Perner S, Verhaak RG et al. SOX2 is an amplified lineage-survival oncogene in lung and esophageal squamous cell carcinomas. Nat Genet 2009; 41: 1238–1242.
Hussenet T, Dali S, Exinger J, Monga B, Jost B, Dembele D et al. SOX2 is an oncogene activated by recurrent 3q26.3 amplifications in human lung squamous cell carcinomas. PLoS One 2010; 5: e8960.
Yuan P, Kadara H, Behrens C, Tang X, Woods D, Solis LM et al. Sex determining region Y-Box 2 (SOX2) is a potential cell-lineage gene highly expressed in the pathogenesis of squamous cell carcinomas of the lung. PLoS One 2010; 5: e9112.
Lu Y, Futtner C, Rock JR, Xu X, Whitworth W, Hogan BL et al. Evidence that SOX2 overexpression is oncogenic in the lung. PLoS One 2010; 5: e11022.
Weiss J, Sos ML, Seidel D, Peifer M, Zander T, Heuckmann JM et al. Frequent and focal FGFR1 amplification associates with therapeutically tractable FGFR1 dependency in squamous cell lung cancer. Sci Transl Med 2010; 2: 62ra93.
Sos ML, Michel K, Zander T, Weiss J, Frommolt P, Peifer M et al. Predicting drug susceptibility of non-small cell lung cancers based on genetic lesions. J Clin Invest 2009; 119: 1727–1740.
Sos ML, Fischer S, Ullrich R, Peifer M, Heuckmann JM, Koker M et al. Identifying genotype-dependent efficacy of single and combined PI3K- and MAPK-pathway inhibition in cancer. Proc Natl Acad Sci USA 2009; 106: 18351–18356.
Marek L, Ware KE, Fritzsche A, Hercule P, Helton WR, Smith JE et al. Fibroblast growth factor (FGF) and FGF receptor-mediated autocrine signaling in non-small-cell lung cancer cells. Mol Pharmacol 2009; 75: 196–207.
Turner NC, Seckl MJ . A therapeutic target for smoking-associated lung cancer. Sci Transl Med 2010; 2: 62ps56.
Dutt A, Ramos AH, Hammerman PS, Mermel C, Cho J, Sharifnia T et al. Inhibitor-Sensitive FGFR1 Amplification in Human Non-Small Cell Lung Cancer. PLoS One 2011; 6: e20351.
Cappellen D, De Oliveira C, Ricol D, de Medina S, Bourdin J, Sastre-Garau X et al. Frequent activating mutations of FGFR3 in human bladder and cervix carcinomas. Nat Genet 1999; 23: 18–20.
Dutt A, Salvesen HB, Chen TH, Ramos AH, Onofrio RC, Hatton C et al. Drug-sensitive FGFR2 mutations in endometrial carcinoma. Proc Natl Acad Sci USA 2008; 105: 8713–8717.
Kunii K, Davis L, Gorenstein J, Hatch H, Yashiro M, Di Bacco A et al. FGFR2-amplified gastric cancer cell lines require FGFR2 and Erbb3 signaling for growth and survival. Cancer Res 2008; 68: 2340–2348.
Turner N, Pearson A, Sharpe R, Lambros M, Geyer F, Lopez-Garcia MA et al. FGFR1 amplification drives endocrine therapy resistance and is a therapeutic target in breast cancer. Cancer Res 2010; 70: 2085–2094.
Sarker D, Molife R, Evans TR, Hardie M, Marriott C, Butzberger-Zimmerli P et al. A phase I pharmacokinetic and pharmacodynamic study of TKI258, an oral, multitargeted receptor tyrosine kinase inhibitor in patients with advanced solid tumors. Clin Cancer Res 2008; 14: 2075–2081.
Hilberg F, Roth GJ, Krssak M, Kautschitsch S, Sommergruber W, Tontsch-Grunt U et al. BIBF 1120: triple angiokinase inhibitor with sustained receptor blockade and good antitumor efficacy. Cancer Res 2008; 68: 4774–4782.
Turner N, Grose R . Fibroblast growth factor signalling: from development to cancer. Nat Rev Cancer 2009; 10: 116–129.
Hammerman PS, Sos ML, Ramos AH, Xu C, Dutt A, Zhou W et al. Mutations in the DDR2 kinase gene identify a novel therapeutic target in squamous cell lung cancer. Cancer Discovery 2011; 1: 78–89.
Ikeda K, Wang LH, Torres R, Zhao H, Olaso E, Eng FJ et al. Discoidin domain receptor 2 interacts with Src and Shc following its activation by type I collagen. J Biol Chem 2002; 277: 19206–19212.
Haura EB, Tanvetyanon T, Chiappori A, Williams C, Simon G, Antonia S et al. Phase I/II study of the Src inhibitor dasatinib in combination with erlotinib in advanced non-small-cell lung cancer. J Clin Oncol 2010; 28: 1387–1394.
Acknowledgements
RKT is supported by the German Ministry of Science and Education (BMBF) as part of the NGFNplus program (grant 01GS08100), by the Max Planck Society (M.I.F.A.NEUR8061 to RKT), by the Deutsche Forschungsgemeinschaft (DFG) through SFB (TP6), the Ministry for Innovation, Science, Research and Technology of the State of Nordrhein-Westfalen (MIWT, 4000- 12 09), by the Fritz-Thyssen-Stiftung (grant 10.08.2.175) and by an anonymous foundation. MLS is supported by an IASLC Lung Cancer Fellowship Award. RKT received consulting and lecture fees (Sanofi-Aventis, Merck, Roche, Boehringer Ingelheim, Astra-Zeneca, Atlas-Biolabs, Johnson&Johnson), as well as research support (AstraZeneca, Merck).
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Competing interests
There is potential conflict of interest.
Rights and permissions
About this article
Cite this article
Sos, M., Thomas, R. Genetic insight and therapeutic targets in squamous-cell lung cancer. Oncogene 31, 4811–4814 (2012). https://doi.org/10.1038/onc.2011.640
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1038/onc.2011.640
Keywords
This article is cited by
-
Non-small-cell lung cancer
Nature Reviews Disease Primers (2015)