-
PDF
- Split View
-
Views
-
Cite
Cite
Vesna Sossi, Raúl de la Fuente-Fernández, Michael Schulzer, John Adams, Jon Stoessl, Age-related differences in levodopa dynamics in Parkinson's: implications for motor complications, Brain, Volume 129, Issue 4, April 2006, Pages 1050–1058, https://doi.org/10.1093/brain/awl028
Close - Share Icon Share
Abstract
Treatment-related motor complications in Parkinson's disease have been previously linked to disease-induced pre-synaptic alterations: dopaminergic denervation and changes in dopamine (DA) release patterns. The occurrence of such complications is also known to be partly dependent on the age of disease onset, occurring more frequently in patients with disease onset at a younger age. Using positron emission tomography (PET) and 4-h-long 18F-fluorodopa (FD) scans we have investigated in vivo an age dependence of disease-induced changes in DA turnover as a possible contributing factor to the age-related differences in treatment-related motor complications. We evaluated the relative changes in DA turnover (measured by its direct inverse, effective DA distribution volume—EDV) and DA synthesis and vesicular storage capacity (quantified by the plasma input uptake rate constant Ki) in Parkinson's disease patients as a function of age (n = 27, age range 38–79 years). After correcting for disease severity, a significant negative correlation was found between age and magnitude of disease-induced decrease in EDV and in Ki in the putamen (P < 0.001, P = 0.02, respectively). However, the difference between the disease-induced decrease in EDV and that in Ki also exhibited an age dependence (P < 0.001), indicating a relatively higher disease-induced increase in DA turnover (inverse of EDV) compared with the decrease in DA synthesis and storage rate in patients of younger age compared with older patients. This finding implies that DA turnover in younger-onset patients undergoes a relatively greater alteration and thus likely contributes to a greater imbalance between DA synthesis, storage and release, which could lead to larger swings in synaptic DA levels. It has indeed been suggested on theoretical grounds that such imbalance may contribute to the greater propensity for motor fluctuations. These results provide one possible explanation for the age-dependent occurrence of complications and support the existence of a pre-synaptic contribution to the occurrence of motor complications.
Introduction
Parkinson's disease is characterized by a progressive degeneration of the dopaminergic system leading to an increasingly more severe dopamine (DA) depletion state. It is known that clinical symptoms, tremor, rigidity and bradykinesia, generally occur when at least 50% of the dopaminergic neurons have died thus implying the existence of a relatively long pre-clinical period during which several disease-induced regulatory and compensatory changes have taken place. At present, all forms of treatment are of symptomatic nature, with the most common being the administration of levodopa. Most patients respond well to levodopa; however, a substantial proportion (∼50%) develop motor complications (motor fluctuations and dyskinesia) within 5 years of chronic levodopa treatment (Marsden and Parkes, 1976; Marsden et al., 1982). Although present in patients of any age at disease onset, the occurrence of treatment-related motor complications has been observed to be more frequent in patients with disease onset at a younger age (Kostic et al., 1991; de la Fuente-Fernandez et al., 2000; Obeso et al., 2000).
The mechanisms underlying such motor complications are as yet not completely understood: the currently favoured hypothesis is the concept that motor complications are related to abnormal pulsatile stimulation of denervated DA receptors by relatively short acting dopaminergic agents such as levodopa. In support of this hypothesis recent studies suggest that motor complications can be reduced with a continuous infusion of levodopa (Stocchi et al., 2005). In this light, motor fluctuations would thus arise from a combined contribution from pre-synaptic and post-synaptic components: altered DA release pattern resulting from nigrostriatal damage and consequent post-synaptic changes due to the resulting pulsatile stimulation of DA receptors.
Recently, a theoretical model describing a possible mechanism for the pre-synaptic involvement in the occurrence of motor complications was developed (de la Fuente-Fernandez et al., 2004a). In this model a probabilistic approach was used to estimate the amount of DA released by exocytosis from the vesicles. The model was able to reproduce the time course of the clinical benefits of treatment for stable responders, wearing-off fluctuators and on–off fluctuators (patients characterized by an increasingly shortened duration of clinical benefits of treatment). The proportion of vesicles that undergo exocytosis per unit time (therefore a measure of DA turnover) was identified as one of the two most important parameters in modelling the time course of levodopa response (together with the re-uptake capacity of surviving terminals). Furthermore the model showed that differences in nigrostriatal DA damage are not strictly necessary to explain the occurrence of motor fluctuations: that is, for equal disease severity a relatively higher turnover is sufficient to induce motor complications. The model also demonstrated that the relative increase in DA release rate necessary to cause fluctuations was lower for a higher level of DA depletion (and thus disease severity).
Further experimental evidence of pre-synaptic involvement in the occurrence of motor complications comes from recent positron emission tomography (PET) studies that showed an altered pattern of exogenous levodopa-derived DA release (an indirect measure of DA turnover) in Parkinson's disease patients who were stable at the time of the PET scans, but who went on to develop motor fluctuations (de la Fuente-Fernandez et al., 2001). These data have shown that such alterations, resulting in a more rapid DA release, precede the occurrence of motor fluctuations thus potentially pointing to an intrinsic ‘predisposition’ towards the onset of motor complications induced by altered DA kinetics, in keeping with the theoretical model. Likewise, it was found that changes in synaptic DA levels after levodopa administration are greater in patients suffering from dyskinesia compared with patients without motor complications (de la Fuente-Fernandez et al., 2004b).
An increased DA turnover associated with Parkinson's disease has been previously found with post-mortem and in vivo studies (Hornykiewicz, 1982; Kuwabara et al., 1993). Recently however it was also shown that a large fraction of this change occurs very early in Parkinson's disease; such increase was interpreted as an early disease-induced compensatory mechanism (Sossi et al., 2002, 2004). Likewise, a relative increase in DA synthesis (and vesicular storage) was also identified as a compensatory mechanism (Lee et al., 2000). It is thus conceivable that a different time course of these regulatory changes as a function of disease progression might lead to a situation where the relationship between the level of DA depletion and DA release rate favours the occurrence of motor complications (de la Fuente-Fernandez et al., 2004a). This hypothesis taken together with the observation that younger-onset patients are more likely to develop treatment-related motor complications prompted the investigation on how disease-related changes in DA turnover are affected by age. We predicted that subjects with disease onset at a younger age might have a relatively greater change in DA turnover compared with the change in DA synthesis and storage rate when compared with subjects with disease onset at an older age.
Methods
Patient selection, scanning protocol and regions of interest (ROI) placement
A group of 10 healthy volunteers [age 58.5 ± 11.2 years (mean ± SD), range 43–76; 5 males (M) and 5 females (F)] was used to investigate the age dependence of the mechanisms under investigation.
The Parkinson's disease subject group comprised 27 patients [age 63.4 ± 9.5 years (mean ± SD), range 38–79; 22 M and 5 F]. The Unified Parkinson's Disease Rating Scale (UPDRS) score, assessed after a minimum of 12 h of all antiparkinson medication, was 27.9 ± 10.8 (mean ± SD), range 11–51. Disease duration from symptom onset was 7.9 ± 5.4 years (range 1–22). Six patients were still untreated at the time of the scan. The other 21 patients were on chronic levodopa treatment, and 11 of them were also taking direct DA agonists [standard levodopa equivalents 913 ± 425 mg/day (mean ± SD)]. There was no correlation between patient age and amount of medication. All medications were stopped the evening before the scan.
All subjects underwent a 4-h-long 18F-fluorodopa (FD) scan on a Siemens/CTI ECAT 953B PET tomograph (Spinks et al., 1992) operating in the 3D mode (Sossi et al., 1998) after injection of 7 mCi of FD (effective dose equivalent 4.2 mSv). All the subjects were administered 150 mg of the peripheral decarboxylase inhibitor carbidopa 1 h prior to FD injection. Data were corrected for attenuation using an external 68Ge source. The scanning sequence consisted of 9 × 10 min scans, after which the patients could leave the bed for 60 min, followed by a final sequence of 9 × 10 min scans. The repositioning was aided by use of a thermoplastic mask, which also helped to minimize head motion during the scans. Images from the first and second scanning sequences were realigned using the AIR algorithm (Woods et al., 1993). Four 61 mm2 circular ROIs were placed on each striatum, one on the caudate nucleus and three on the putamen [anterior (P1), middle (P2) and posterior putamen (P3)], and six 270 mm2 circular ROIs were placed on the occipital cortex on five adjacent planes containing the striatal image on each frame of the dynamic sequence.
For each study, 37 arterial blood samples were taken to obtain the plasma radioactivity time course and 12 were analysed for metabolites to identify the authentic FD plasma fraction. The metabolite separation was performed as previously described with an alumina extraction method with anion/cation exchange columns (Doudet et al., 1998; McLellan et al., 1991). With this method, the cation column captures the positively charged metabolites [18F-fluorodopamine (18F-DA) and 6–fluoro-3-methoxytyramine] and the anion column the negatively charged metabolites [L-3,4-dihydroxy-6-[18F]fluorophenylacetic acid (FDOPAC), [18F]6-fluoro-homovanillic acid (FHVA) and sulphated conjugates]. FD and 3-O-methylfluorodopa (3OMFD), not retained by the columns, are then separated by alumina extraction. All subjects gave written informed consent. The study received the approval of the University of British Columbia Ethics Committee.
Modelling
After tracer injection, FD is converted into (18F-DA) and stored into synaptic vesicles. The DA synthesis and vesicular storage rate constant (FD uptake rate constant) Ki was determined using the plasma slope-intercept graphical approach (Gjedde, 1981, 1982, Patlak et al., 1983) from the first 90 min of data [Ki = K1k3/(k2 + k3)], where K1 and k2 are the clearance rate constants from plasma into tissue and from tissue into plasma, respectively, and k3 is the rate constant describing the trapping of brain FD. In order to eliminate the contribution of the FD metabolite 3OMFD, the radioactivity present in an ROI placed on the occipital cortex was subtracted from the radioactivity determined in an ROI placed on the striatal regions (Martin et al., 1989). While during the first 90 min after injection the data defined by the graphical analysis generally follow a straight line (except for very advanced patients), at times later than ∼90–120 min after tracer injection the data start to deviate from a straight line, indicating some degree of reversibility. This reversibility is considered primarily due to DA release and subsequent metabolism, either synaptic or cytoplasmic following DA re-uptake in the pre-synaptic terminal. It is likely that some intracytoplasmic metabolism occurs before the initial storage of DA (i.e. when DA is synthesized). However, this process should not affect the turnover measurement, since this fraction of DA is never trapped. A reversible tracer approach model was thus applied to the data acquired between 150 and 240 min post-injection to determine effective DA distribution volume (EDV). We have previously shown that the EDV estimated in this way is mathematically and conceptually equal to Ki/kloss, where the rate constant kloss quantifies the degree of tracer reversibility (Sossi et al., 2001).
EDV is the direct inverse of the effective DA turnover (Doudet et al., 1998) and the two terms are thus used interchangeably. Statistical analysis however was performed on EDV, since EDV is the quantity directly measured. Thus, while Ki is primarily a measure of DA synthesis rate (thus dopa decarboxylase activity) and initial vesicular storage (thus terminal density), DA turnover provides additional information on the neuronal DA release rate.
Data analysis
Radiotracer concentrations in the caudate nucleus and putamen were estimated by averaging the values in the two caudate ROIs and in the six putaminal ROIs, and Ki and EDV were calculated from the resulting time courses.
Age dependence of EDV and Ki in control subjects
Regression analysis was used to derive the age relation in normal controls for Ki and EDV for the caudate and putamen separately.
Age dependence of disease-induced changes in Ki and EDV (Kiputdiff and EDVputdiff) in Parkinson's disease patients
Once the age relation was established in the normal controls (significance was reached only for the putamen—see Results) the difference between the observed putaminal Ki and EDV values for each patient and the corresponding age-matched values in the normal controls (Kiputdiff and EDVputdiff) were calculated. Such differences, intrinsically negative since representing disease-induced changes, were then further regressed on subject age. We also tested for non-linear effects in age. Multiple regression analysis was performed on age and disease duration (defined as the time elapsed since onset of clinical symptoms) and age and UPDRS, where UPDRS was considered to be a clinical measure of disease severity. EDVputdiff was also regressed on age and Ki putamen (Kiput), where Kiput was taken to represent an alternative measure of disease severity.
Relative age dependence of EDVputdiff versus Kiputdiff
In order to test whether the relative magnitude (and thus presumably the time course) of these changes varied as a function of subject age, it was first necessary to express EDVputdiff and Kiputdiff in commensurate units.
This was achieved by standardizing these measurements, by dividing them by their corresponding standard errors (SEs), EDV*putdiff = EDVputdiff/SE(EDVputdiff) and K*iputdiff = Kiputdiff/se(Kiputdiff). However, as the directly estimated SEs reflected also the effects of inter-subject variation in age and in severity, age- and UPDRS-adjusted SEs were calculated by means of multiple regressions. The resulting SEs of the regressions represented the pure inter-subject variation in the measurements controlled for age and severity. To relate these standardized values of EDV*putdiff and K*iputdiff to age and UPDRS, bivariate multiple regression was carried out, as these two sets of standardized variables were highly correlated within patients. The slopes on subject age were compared. For an even more direct comparison, the variable DIFF, defined as EDV*putdiff−K*iputdiff, was regressed on age and UPDRS.
Results
Age dependence of EDV and Ki in control subjects
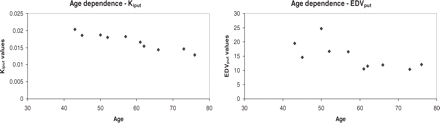
Linear regression was found to best describe the data in all cases. A significant age dependence (negative correlation) was found for EDV and Ki for the putamen (P = 0.03 and P < 0.0001, respectively; Fig. 1). No significant age dependence was found for the caudate nucleus for either variable (P = 0.32 and 0.09, respectively). Further analysis was thus performed for the putamen only (Kiput and EDVput).
Age dependence for Kiput (left) and EDVput (right) in healthy controls.
Age dependence of disease-induced changes in Kiput and EDVput (Kiputdiff and EDVputdiff) in Parkinson's disease patients
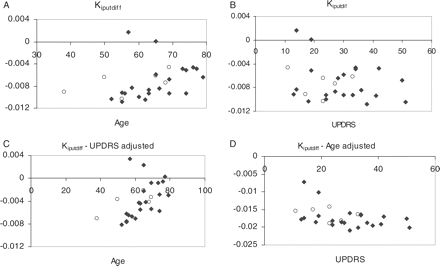
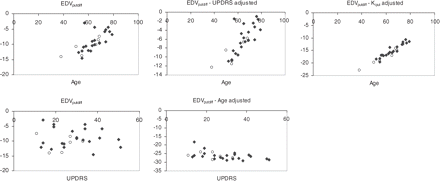
The significance levels for each regression and the regression coefficients are shown in Table 1, while the scatter-plots associated with the regression analysis are shown in Figures 2 and 3. Regression of Kiputdiff on age or UPDRS alone did not reach statistical significance (P = 0.10 and 0.24, respectively); however, when both age and UPDRS were included in the regression, statistical significance was reached for both variables (P = 0.02 and 0.04, respectively). On the other hand, the regression of EDVputdiff on age was highly significant (P < 0.0001), unlike the regression on UPDRS alone (P = 0.84). When a multiple regression on age and UPDRS was performed, the contribution from both variables became significant (P < 0.0001 and P = 0.02). Multiple regression on age and on Kiput taken as an index of disease severity showed the contribution from both variables to be highly significant (P < 0.0001 for both variables). Dependence on disease duration was found to be not significant for either variable.
Kiputdiff age and UPDRS dependence for the Parkinson's disease subjects—raw and adjusted values. Adjusted values were plotted when the Kiputdiff dependence on the particular variable was found to be significant. Open circles = untreated subjects.
EDVputdiff age and UPDRS dependence for the Parkinson's disease subjects—raw and adjusted values. Adjusted values were plotted when the Kiputdiff dependence on the particular variable was found to be significant. Open circles = untreated subjects.
Results from the statistical analysis for Kiputdiff and EDVputdiff
| Variable . | Independent variable . | Individual P-value . | Regression coefficients . | . | Overall P-value . | |
|---|---|---|---|---|---|---|
. | . | . | Slope . | Intercept . | . | |
| Kiputdiff | Age | 0.10 | 0.104 × 10−3 | −0.0138 | 0.10 | |
| UPDRS | 0.24 | 0.067 × 10−3 | −0.0053 | 0.24 | ||
| Age | 0.95 | −0.415 × 10−4 | −0.0095 | 0.26 | ||
| Age2 | 0.81 | 0.119 × 10−5 | ||||
| Dur | 0.53 | −0.732 × 10−4 | −0.0141 | 0.223 | ||
| Age | 0.09 | 0.118 × 10−3 | ||||
| UPDRS | 0.04 | −0.120 × 10−3 | −0.0138 | 0.03 | ||
| Age | 0.02 | 0.157 × 10−3 | ||||
| EDVputdiff | Age | <0.0001 | 0.2213 | −23.41 | <0.0001 | |
| UPDRS | 0.84 | −0.0118 | −9.04 | 0.84 | ||
| Age | 0.95 | −0.031 | −15.90 | <0.0001 | ||
| Age2 | 0.60 | 0.0021 | ||||
| Dur | 0.46 | −0.0694 | −23.71 | <0.0001 | ||
| Age | <0.0001 | 0.2346 | ||||
| UPDRS | 0.02 | −0.1024 | −23.43 | <0.0001 | ||
| Age | <0.0001 | 0.266 | ||||
| Kiput | <0.0001 | 741.63 | −34.41 | <0.0001 | ||
| Age | <0.0001 | 0.295 | ||||
| Variable . | Independent variable . | Individual P-value . | Regression coefficients . | . | Overall P-value . | |
|---|---|---|---|---|---|---|
. | . | . | Slope . | Intercept . | . | |
| Kiputdiff | Age | 0.10 | 0.104 × 10−3 | −0.0138 | 0.10 | |
| UPDRS | 0.24 | 0.067 × 10−3 | −0.0053 | 0.24 | ||
| Age | 0.95 | −0.415 × 10−4 | −0.0095 | 0.26 | ||
| Age2 | 0.81 | 0.119 × 10−5 | ||||
| Dur | 0.53 | −0.732 × 10−4 | −0.0141 | 0.223 | ||
| Age | 0.09 | 0.118 × 10−3 | ||||
| UPDRS | 0.04 | −0.120 × 10−3 | −0.0138 | 0.03 | ||
| Age | 0.02 | 0.157 × 10−3 | ||||
| EDVputdiff | Age | <0.0001 | 0.2213 | −23.41 | <0.0001 | |
| UPDRS | 0.84 | −0.0118 | −9.04 | 0.84 | ||
| Age | 0.95 | −0.031 | −15.90 | <0.0001 | ||
| Age2 | 0.60 | 0.0021 | ||||
| Dur | 0.46 | −0.0694 | −23.71 | <0.0001 | ||
| Age | <0.0001 | 0.2346 | ||||
| UPDRS | 0.02 | −0.1024 | −23.43 | <0.0001 | ||
| Age | <0.0001 | 0.266 | ||||
| Kiput | <0.0001 | 741.63 | −34.41 | <0.0001 | ||
| Age | <0.0001 | 0.295 | ||||
Significant P-values are given in boldface.
Results from the statistical analysis for Kiputdiff and EDVputdiff
| Variable . | Independent variable . | Individual P-value . | Regression coefficients . | . | Overall P-value . | |
|---|---|---|---|---|---|---|
. | . | . | Slope . | Intercept . | . | |
| Kiputdiff | Age | 0.10 | 0.104 × 10−3 | −0.0138 | 0.10 | |
| UPDRS | 0.24 | 0.067 × 10−3 | −0.0053 | 0.24 | ||
| Age | 0.95 | −0.415 × 10−4 | −0.0095 | 0.26 | ||
| Age2 | 0.81 | 0.119 × 10−5 | ||||
| Dur | 0.53 | −0.732 × 10−4 | −0.0141 | 0.223 | ||
| Age | 0.09 | 0.118 × 10−3 | ||||
| UPDRS | 0.04 | −0.120 × 10−3 | −0.0138 | 0.03 | ||
| Age | 0.02 | 0.157 × 10−3 | ||||
| EDVputdiff | Age | <0.0001 | 0.2213 | −23.41 | <0.0001 | |
| UPDRS | 0.84 | −0.0118 | −9.04 | 0.84 | ||
| Age | 0.95 | −0.031 | −15.90 | <0.0001 | ||
| Age2 | 0.60 | 0.0021 | ||||
| Dur | 0.46 | −0.0694 | −23.71 | <0.0001 | ||
| Age | <0.0001 | 0.2346 | ||||
| UPDRS | 0.02 | −0.1024 | −23.43 | <0.0001 | ||
| Age | <0.0001 | 0.266 | ||||
| Kiput | <0.0001 | 741.63 | −34.41 | <0.0001 | ||
| Age | <0.0001 | 0.295 | ||||
| Variable . | Independent variable . | Individual P-value . | Regression coefficients . | . | Overall P-value . | |
|---|---|---|---|---|---|---|
. | . | . | Slope . | Intercept . | . | |
| Kiputdiff | Age | 0.10 | 0.104 × 10−3 | −0.0138 | 0.10 | |
| UPDRS | 0.24 | 0.067 × 10−3 | −0.0053 | 0.24 | ||
| Age | 0.95 | −0.415 × 10−4 | −0.0095 | 0.26 | ||
| Age2 | 0.81 | 0.119 × 10−5 | ||||
| Dur | 0.53 | −0.732 × 10−4 | −0.0141 | 0.223 | ||
| Age | 0.09 | 0.118 × 10−3 | ||||
| UPDRS | 0.04 | −0.120 × 10−3 | −0.0138 | 0.03 | ||
| Age | 0.02 | 0.157 × 10−3 | ||||
| EDVputdiff | Age | <0.0001 | 0.2213 | −23.41 | <0.0001 | |
| UPDRS | 0.84 | −0.0118 | −9.04 | 0.84 | ||
| Age | 0.95 | −0.031 | −15.90 | <0.0001 | ||
| Age2 | 0.60 | 0.0021 | ||||
| Dur | 0.46 | −0.0694 | −23.71 | <0.0001 | ||
| Age | <0.0001 | 0.2346 | ||||
| UPDRS | 0.02 | −0.1024 | −23.43 | <0.0001 | ||
| Age | <0.0001 | 0.266 | ||||
| Kiput | <0.0001 | 741.63 | −34.41 | <0.0001 | ||
| Age | <0.0001 | 0.295 | ||||
Significant P-values are given in boldface.
Relative age dependence of EDVputdiff versus Kiputdiff
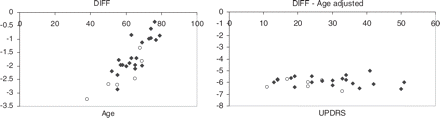
The multiple regression analysis provided the following regression coefficients: K*iputdiff = −5.006 + 0.0569 age − 0.0436 UPDRS (age coefficient P < 0.0001, and UPDRS coefficient P = 0.0107) and EDV*putdiff = −10.821 + 0.1231 age − 0.0473 UPDRS (age coefficient P = 0.0071 and UPDRS coefficient P = 0.0187). The age-related regression coefficients are significantly different (P = 0.001), indicating an approximately double age effect for EDVputdiff compared with Kiputdiff. The multiple regression for DIFF yielded DIFF = −5.814 + 0.0662 age − 0.0037 UPDRS, where the age coefficient had P < 0.0001 and the UPDRS coefficient had P = 0.65 (Fig. 4).
DIFF (EDV*putdiff − K*iputdiff) dependence on function of age (left) and age adjusted DIFF dependence on UPDRS (right). Adjusted values were plotted when the DIFF dependence on the particular variable was found to be significant. Open circles = untreated subjects.
Discussion
Age dependence of EDV and Ki in control subjects
This study shows in vivo a significant decrease of the EDV (and consequent increase in DA turnover) as a function of age using PET and a direct analysis method to determine EDV. These results are in agreement with post-mortem studies (Haycock et al., 2003) and with recent PET imaging results where an EDV was calculated from a separate determination of a modified Ki and kloss (Kumakura et al., 2005). Such increase could be compensatory in nature, where DA turnover (DA release) increases to compensate for declining DA levels (Kish et al., 1992; Haycock et al., 2003) arising as a consequence of the age-related loss of the dopaminergic neurons (McGeer et al., 1977; Fearnley and Lees, 1991). In addition, an age-related increase of the catecholamine-oxidizing enzyme monoamine oxidase-B (MAO-B) may also contribute to a declining capacity to retain 18F-DA within the brain for extended periods (Fowler et al., 1997; Kumar and Andersen, 2004).
The effect of normal ageing on Ki is more controversial. While there seems to be a general consensus about age-related loss of dopaminergic neurons in human post-mortem studies (McGeer et al., 1977; Fearnley and Lees, 1991), PET studies involving measures of DA synthesis and storage have given inconsistent conclusions, with some reports failing to reveal a significant decline (Sawle et al., 1990; Eidelberg et al., 1993) and others showing a decline (Martin et al., 1989; Cordes et al., 1994). Part of the reason could be the use of different analysis methods that have different sensitivity to issues such as presence of the FD metabolite 3OMFD or to kloss, (Sossi et al., 2003; Kumakura et al., 2005), possible age-dependent behaviour of the selected reference region (Sossi et al., 2005; Kops et al., 2002) or age range of the subjects included in the study (Haycock et al., 2003). However the age dependence of Kiput found in this study was extremely robust (P < 0.0001) and the age range of the normal volunteers included in this study spanned almost the entire age range of the Parkinson's disease patients considered. Results from the regression analysis suggest a loss of 0.7% per year of extrapolated Kiput at birth. This value is in good agreement with human post-mortem studies on the loss of dopaminergic neurons from the substantia part compacta, indicating 0.5–0.7% per year reduction (McGeer et al., 1977; Fearnley and Lees, 1991). In addition, Frey et al. (1996) have found a very similar (0.7%) age-related loss rate for specific binding of the vesicular monoamine transporter marker [11C](+)-dihydrotetrabenazine using PET. Although FD Ki and DTBZ specific binding trace two different (although related) aspects of the dopaminergic system, it is not surprising that, in the absence of age-related regulatory changes, the values of both parameters remain proportional to the number of existing neurons and thus change at a similar rate as a function of age. Results from a similar estimate derived from the regression analysis show a loss of 0.9% per year of extrapolated EDVput at birth. This value is slightly higher compared with that obtained for Kiput, likely reflecting not only the effect of age-related neuronal loss but also the age-related increase in MAO-B.
Age dependence of disease-induced changes in Ki and EDV: (Kiputdiff and EDVputdiff) in Parkinson's disease patients
It is interesting to notice that for Parkinson's disease patients the Kiputdiff achieved a significant age dependence only when its values were adjusted for the UPDRS scores, implying that both disease severity and age contribute significantly to the decrease of Kiput values. In particular, a comparison of Fig. 2B and D emphasizes the much more consistent dependence of Kiputdiff on UPDRS, once the data are adjusted for the age effect. For Parkinson's disease patients there was no age dependence in the Kiput values themselves once adjusted for UPDRS (P = 0.47) (while there is a significant dependence on UPDRS alone, P = 0.04), indicating that age of onset does not influence the absolute severity of nigrostriatal damage, in agreement with previous reports (de la Fuente-Fernandez et al., 2003). Taken together, these two findings indicate that the change from an age-matched baseline is greater in younger-onset patients compared with those with disease onset at an older age evaluated at equal disease severity as estimated by clinical measures. If one assumes similar disease mechanisms in younger- and older-onset patients, younger-onset patients might thus be experiencing a longer pre-clinical period. Invoking the further assumption of a non-linear time dependence of disease progression (Lee et al., 2004) one might speculate that the observed relative slower progression of disease severity in younger patients could reflect the fact that they are indeed at a later stage of disease relative to its onset.
In contrast to Kiputdiff, EDVputdiff shows a significant age dependence even when disease severity, evaluated either clinically by UPDRS scores or by Kiput, is not taken into account (P < 0.0001). Multiple regression analysis however demonstrates an additional significant dependence on either UPDRS or Kiput. As observed for Kiput, there is a lack of age dependence for the EDVput values themselves in Parkinson's disease (P = 0.71), which are more highly related to UPDRS (P = 0.02). Again, these findings emphasize the fact that the absolute disease severity is independent of the age of onset, while, in this case, the change from age-matched baseline is very highly dependent on age.
The fact that neither Kiputdiff nor EDVputdiff depends significantly on duration of clinical symptoms can be interpreted as further evidence that clinical symptoms might appear at different time-stages of actual neuronal degeneration for different ages of onset.
Relative age dependence of EDVputdiff versus Kiputdiff
The analysis of EDV*putdiff and K*iputdiff showed the effect of age to be approximately twice as great for EDV*putdiff compared with K*iputdiff, while interestingly the regression coefficients for UPDRS were very similar. This is consistent with previous results (Sossi et al., 2004) showing that as disease progresses the relative magnitude of changes in Ki and EDV become comparable. The results of the DIFF analysis confirm this finding (Fig. 4).
Since both Kiputdiff and EDVputdiff represent disease-specific changes (i.e. their expected value in the absence of disease is zero), these results would suggest that the differential effect of age on Kiputdiff and EDVputdiff indeed uncovers age-related differences in the way patients handle Parkinson's disease pathology. The finding that the change in EDVputdiff compared with Kiputdiff (i.e. DIFF) is greater in Parkinson's disease patients with disease onset at a younger age indicates that the increase in DA turnover compared with age-matched normal conditions is relatively greater in younger people relative to the decrease in their ability to synthesize and package DA into synaptic vesicles. While in the very early stages of the disease such changes might act as compensatory mechanisms to maintain quasi-normal synaptic DA levels and thus prove beneficial, as disease progresses the younger-onset subjects are more likely to experience large swings in synaptic DA levels. According to the theoretical model described above (de la Fuente-Fernandez et al., 2004) such conditions would be exactly those required to increase the probability of motor fluctuations. Interestingly, in a study that showed alteration in DA release rate to precede the occurrence of motor fluctuations (de la Fuente-Fernandez et al., 2001a) the four patients, who at the time of PET scanning showed a faster DA release and consequently developed motor fluctuations, were indeed younger.
While this study provides experimental evidence for a pre-synaptic basis for the occurrence of motor complications by indirectly examining changes in DA release rate, it is not in disagreement with the hypothesis of a post-synaptic contribution to these phenomena. A greater disease-induced increase in DA turnover is necessarily associated with more severely altered DA release patterns. Thus, when administered exogenous therapeutic levodopa such change in DA release pattern might lead to a more pulsatile-like behaviour of the synaptic DA concentration, which, as previously argued, might lead to post-synaptic receptor changes associated with the occurrence of motor complications. However, as pointed out previously (de la Fuente-Fernandez et al., 2004a), these results would also suggest that levodopa therapy by itself is likely not the cause of motor complication. In fact, a closer look at the plots shown in Figs 2–4 reveals that all the variable-specific values encountered in the untreated group are completely consistent with those observed in treated patients. Rather, age dependency of regulatory changes associated with Parkinson's disease pathology may contribute to the different time-response to levodopa treatment often observed as a function of age of onset. It is also possible to speculate that part of the reason why younger-onset patients initially respond to levodopa therapy better (Granérus et al., 1979) might indeed be the relatively larger increase in turnover and thus more rapid and efficient DA utilization.
A final note of caution is in order. The findings reported in this study depend crucially on the existence of an age dependence of DA turnover in the normal population. However, in addition to our own results, data in the literature seem to support such dependence in a rather robust fashion both in human and animal studies (Barrio et al., 1990; Haycock et al., 2003; Kumakura et al., 2005), thus lending support to our conclusions. While there is more controversy surrounding the age dependence of Ki, our fundamental conclusions would only be strengthened by lack of such age dependency, by increasing the disparity of the magnitude of EDVputdiff and Kiputdiff as a function of age.
Conclusions
This study demonstrated in vivo that the degree of abnormality in DA turnover and DA synthesis and storage rate depend upon the age of disease onset. These results thus suggest an age-related difference in the way patients respond to the pathology of Parkinson's disease. In particular, in younger-onset patients the relative difference between the abnormality in DA turnover and that in DA synthesis and storage rate change is greater compared with older-onset patients. Such conditions, which have been previously theoretically hypothesized to increase the probability of motor complications, might explain the greater propensity towards such complications clinically observed in patients with disease onset at an younger age.
The authors wish to thank the Director of the PET centre Dr Thomas Ruth, Dr J. Holden for the helpful discussion, Dr Ajit Kumar for patient assessment, Mr Edwin Mak for help with the statistical analysis, Mss Linda Grantier and Sharon Yardley for patient information and the UBC/TRIUMF PET group for their technical support. This work was supported by the National Science and Research Council (V.S.), the Michael Smith Foundation for Health Research (V.S.), a Canada Research Chair (A.J.S.), the Canadian Institutes for Health Research and a Triumf Life Science grant.
References
Barrio JR, Huang SC, Melega WP, Yu DC, Hoffman JM, Schneider JS, et al. 6-[18F]fluoro-L-dopa probes dopamine turnover rates in central dopaminergic structures.
Cordes M, Snow BJ, Cooper S, Schulzer M, Pate BD, Ruth TJ, et al. Age dependent decline of nigrostriatal dopaminergic function: a positron emission tomographic study of grandparents and their grandchildren.
de la Fuente-Fernández R, Pal PK, Vingerhoets FJG, Kishore A, Schulzer M, Mak EK, et al. Evidence for impaired presynaptic dopamine function in parkinsonian patients with motor fluctuations.
de la Fuente-Fernández R, Lu J-Q, Sossi V, Jivan S, Schulzer M, Holden JE, et al. Biochemical variations in the synaptic level of dopamine precede motor fluctuations in Parkinson's disease: PET evidence of increased dopamine turnover.
de la Fuente-Fernandez R, Lim AS, Sossi V, Adam MJ, Ruth TJ, Calne DB, et al. Age and severity of nigrostriatal damage at onset of Parkinson's disease.
de la Fuente-Fernández R, Schulzer M, Mak E, Calne DB, Stoessl A. Presynaptic mechanisms of motor fluctuations in Parkinson's disease: a probabilistic model.
de la Fuente-Fernandez R, Sossi V, Huang Z, Furtado S, Lu JQ, Calne DB, et al. Levodopa-induced changes in synaptic dopamine levels increase with progression of Parkinson's disease: implications for dyskinesias.
Doudet DJ, Chan G, Holden JE, McGeer EG, Aigner TA, Wyatt RJ, et al. 6-[18F]Fluoro-L-DOPA PET studies of the turnover of dopamine in MPTP-induced parkinsonism in monkeys.
Eidelberg D, Takikawa S, Dhawan V, Chaly T, Robeson W, Dahl R, et al. Striatal 18F-dopa uptake: absence of an aging effect.
Fearnley JM, Lees AJ. Ageing and Parkinson's disease: substantia nigra regional selectivity.
Fowler JS, Volkow ND, Wang GJ, Logan J, Pappas N, Shea C, et al. Age-related increases in brain monoamine oxidase B in living healthy human subjects.
Frey KA, Koeppe RA, Kilbourn MR, Vander Borght TM, Albin RL, Gilman S, et al. Presynaptic monoaminergic vesicles in Parkinson's disease and normal aging.
Gjedde A. High- and low-affinity transport of D-glucose from blood to brain.
Gjedde A. Calculation of glucose phoshorylation from brain uptake of glucose analogues in vivo: a re-examination.
Granérus AK, Carlsson A, Svanborg A. The aging neuron—influence on symptomatology and therapeutic response in Parkinson's syndrome. In: Poirier LJ, Sourkes TL, Bedard PJ, editors. Advances in neurology. Vol. 24, New York: Raven Press;
Haycock JW, Becker L, Ang L, Furukawa Y, Hornykiewicz O, Kish SJ. Marked disparity between age-related changes in dopamine and other presynaptic dopaminergic markers in human striatum.
Hornykiewicz O. Brain neurotransmitter changes in Parkinson's disease. In: Marsden CD, Fahn S, editors. Movement disorders. London: Butterworth;
Kish SJ, Shannak K, Rajput A, Deck JHN, Hornykiewicz O. Aging produces a specific pattern of striatal dopamine loss: implications for the etiology of idiopathic Parkinson's disease.
Kops ER, Storb SH, Herzog HR, Bauer A. Age dependency of cortex values before and after partial volume correction in PET data. Nuclear Science Symposium Conference Record. IEEE Vol. 2:
Kostic V, Przedborski S, Flaster E, Sternic N. Early development of levodopa-induced dyskinesias and response fluctuations in young-onset Parkinson's disease.
Kumakura Y, Vernaleken I, Grunder G, Bartenstein P, Gjedde A, Cumming P. PET studies of net blood-brain clearance of FDOPA to human brain: age-dependent decline of [18F]fluorodopamine storage capacity.
Kumar MJ, Andersen JK. Perspectives on MAO-B in aging and neurological disease: where do we go from here?
Kuwabara H, Cumming P, Yasuhara Y, Leger GC, Guttman M, Diksic M, et al. Dopamine turnover is increased in striatum of patients with Parkinson's disease. Brain
Lee CS, Samii A, Sossi V, Ruth TJ, Schulzer M, Holden JE, et al. In vivo positron emission tomographic evidence for compensatory changes in presynaptic dopaminergic nerve terminals in Parkinson's disease.
Lee CS, Schulzer M, de la Fuente-Fernandez R, Mak E, Kuramoto L, Sossi V, et al. Lack of regional selectivity during the progression of Parkinson disease: implications for pathogenesis.
Marsden CD, Parkes JD, Quinn N. Fluctuations of disability in Parkinson's disease - clinical aspects. In: Marsden CD, Fahn S, editors. Movement disorders. London: Butterworth;
Marsden CD, Parkes JD. On-off effects in patients with Parkinsons disease on chronic levodopa therapy.
Martin WRW, Palmer MR, Patlak CS, Calne DB. Nigrostriatal function in man studied with positron emission tomography.
McLellan CA, Doudet DJ, Bruecke T, Aigner TG, Cohen RM, New rapid analysis method demonstrates differences in 6-[18F]fluoro-L-DOPA input curves with and without carbidopa and in hemi-MPTP lesioned monkeys.
Obeso JA, Rodrigues-Oroz MC, Chana P, Lera G, Rodriguez M, Olanow CW. The evolution and origin of motor complications in Parkinson's disease.
Patlak CS, Blasberg RG, Fenstermacher JD. Graphical evaluation of blood-to-brain transfer constants from multiple-time uptake data.
Sawle GV, Colebatch JG, Shah A, Brooks DJ, Marsden CD, Frackowiak RS. Striatal function in normal aging: implications for Parkinson's disease.
Sossi V, Oakes TR, Chan GL, Schulzer M, Ruth TJ. Quantitative comparison of three- and two-dimensional PET with human brain studies.
Sossi V, Doudet DJ, Holden JE. A reversible tracer analysis approach to the study of effective dopamine turnover.
Sossi V, de la Fuente-Fernãndez R, Holden JE, Doudet DJ, McKenzie J, Stoessl AJ, et al. Increase in dopamine turnover occurs early in Parkinson's disease: evidence from a new modeling approach to PET 18F-fluorodopa data. J Cereb Blood Flow and Metab
Sossi V, Holden JE, de la Fuente-Fernandez R, Ruth TJ, Stoessl AJ. Effect of dopamine loss and the metabolite 3-O-methyl-[18F]fluoro-dopa on the relation between the 18F-fluorodopa tissue input uptake rate constant Kocc and the [18F]fluorodopa plasma input uptake rate constant Ki.
Sossi V, de la Fuente-Fernandez R, Holden JE, Schulzer M, Ruth TJ, Stoessl J. Changes of dopamine turnover in the progression of Parkinson's disease as measured by positron emission tomography: their relation to disease-compensatory mechanisms.
Sossi V, Holden JE, Schulzer M, Ruth TJ, Stoessl AJ. Age dependence of 18F-fluorodopa uptake rate and dopamine turnover PET markers and implications for Parkinson's disease progression. In: Proceedings of the XXIInd International Symposium on Cerebral Blood Flow, Metabolism, and Function, June 7-11, 2005, Amsterdam, The Netherlands, CDROM.
Spinks TJ, Jones T, Bailey DJ, Townsend DW, Grootoonk S, Bloomfield PM, et al. Physical performance of a positron tomograph for brain imaging with retractable septa.
Stocchi F, Vacca L, Ruggieri S, Olanow CW. Intermittent vs continuous levodopa administration in patients with advanced Parkinson disease: a clinical and pharmacokinetic study.
Author notes
1University of British Columbia, 2Pacific Parkinson's Research Centre, Vancouver, Canada and 3Division of Neurology, Hospital Arquitecto Marcide, 15405 Ferrol (A Coruña), Spain