-
PDF
- Split View
-
Views
-
Cite
Cite
Heiko Mahrholdt, Anja Wagner, Robert M. Judd, Udo Sechtem, Raymond J. Kim, Delayed enhancement cardiovascular magnetic resonance assessment of non-ischaemic cardiomyopathies, European Heart Journal, Volume 26, Issue 15, August 2005, Pages 1461–1474, https://doi.org/10.1093/eurheartj/ehi258
Close - Share Icon Share
Abstract
Non-ischaemic cardiomyopathies (NICMs) are chronic, progressive myocardial diseases with distinct patterns of morphological, functional, and electrophysiological changes. In the setting of cardiomyopathy (CM), determining the exact aetiology is important because the aetiology is directly related to treatment and patient survival. Determining the exact aetiology, however, can be difficult using currently available imaging techniques, such as echocardiography, radionuclide imaging or X-ray coronary angiography, since overlap of features between CMs may be encountered.
Cardiovascular magnetic resonance (CMR) imaging has recently emerged as a new non-invasive imaging modality capable of providing high-resolution images of the heart in any desired plane. Delayed contrast enhanced CMR (DE-CMR) can be used for non-invasive tissue characterization and may hold promise in differentiating ischaemic from NICMs, as the typical pattern of hyperenhancement can be classified as ‘ischaemic-type’ or ‘non-ischaemic type’ on the basis of pathophysiology of ischaemia.
This article reviews the potential of DE-CMR to distinguish between ischaemic and NICM as well as to differentiate non-ischaemic aetiologies. Rather than simply describing various hyperenhancement patterns that may occur in different disease states, our goal will be (i) to provide an overall imaging approach for the diagnosis of CM and (ii) to demonstrate how this approach is based on the underlying relationships between contrast enhancement and myocardial pathophysiology.
Introduction
Non-ischaemic cardiomyopathies (NICMs) are chronic, progressive myocardial diseases with distinct patterns of morphological, functional, and electrophysiological changes. On the basis of clinical and morphological findings the World Health Organisation (WHO) has classified cardiomyopathies (CMs) in separate categories such as dilated CM (DCM), hypertrophic CM (HCM) with and without obstruction, and restrictive CM (RCM).1 Although those CMs defined by the WHO were originally understood as being ‘primary’, several etiologic factors leading to the different phenotypes have been identified. A genetic disposition with a possible additional effect of inflammatory or toxic injuries to the myocardium may contribute to the pathogenesis of DCM.2 Genetic defects are also related to HCM3 and all morphologic types of CMs can result from systemic, infiltrative, and storage disorders such as amyloidosis, sarcoidosis, or Anderson–Fabry disease.
In the setting of CM, determining the exact aetiology is important because the aetiology is directly related to treatment and patient survival.4 Therapeutic options include corticosteroids in the setting of cardiac sarcoidosis or certain forms of myocarditis, alkyloids for treatment of cardiac amyloidosis, and septal ablation or myectomy for HCM. However, determining the exact aetiology can be difficult using currently available imaging techniques, such as echocardiography, radionuclide imaging, or X-ray coronary angiography, as overlap of features between CMs may be encountered (Table 1). Even endomyocardial biopsy, which is the current gold standard for determining the aetiology of a variety of NICMs, suffers from limitations such as sampling error and poor sensitivity.5
Cardiovascular magnetic resonance (CMR) imaging has recently emerged as a new non-invasive imaging modality capable of providing high-resolution images of the heart in any desired plane and without radiation. Rather than a single technique, CMR consists of several techniques that can be performed separately or in various combinations during a patient examination. For example, cine-CMR can provide assessment of cardiac morphology and function, first-pass contrast-enhanced perfusion-CMR with and without vasodilators can provide assessment of myocardial perfusion reserve, and delayed contrast enhanced CMR (DE-CMR) can be used for non-invasive tissue characterization.
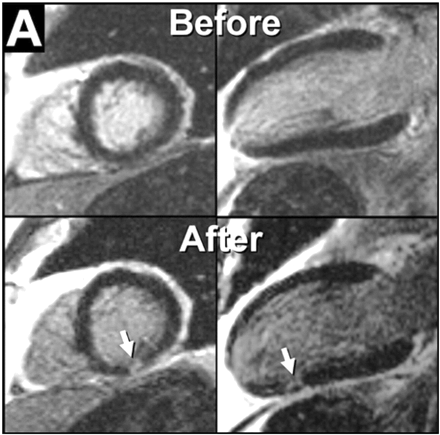
DE-CMR has undergone substantial recent improvement,6 and studies have demonstrated that this technique is effective in detecting the presence, location, and extent of myocardial infarction, and predicting improvement in contractile function following revascularization in both the acute and chronic settings.7 Moreover, DE-CMR is capable of visualizing micro scars that cannot be detected by other imaging techniques (Figure 1).8,9
This article reviews the potential of DE-CMR to distinguish between ischaemic and NICM as well as to differentiate non-ischaemic aetiologies. Rather than simply describing various hyperenhancement patterns that may occur in different disease states, our goal will be (i) to provide an overall imaging approach for the diagnosis of CM and (ii) to demonstrate how this approach is based on the underlying relationships between hyperenhancement and myocardial pathophysiology.
Differentiation between ischaemic and NICM
One of the questions that arises when a patient presents for work-up of left ventricular (LV) dysfunction is whether the dysfunction is ischaemic or non-ischaemic in aetiology. Thus, in many centres coronary angiography is routinely performed to answer this question. In those patients without significant coronary artery disease (CAD), the diagnosis of NICM is usually made. This differentiation is clinically important as ischaemic CM (ICM) is associated with shorter mean survival than NICM.10 In addition, patient management is altered as patients with ischaemic heart disease may benefit from revascularization, aneurysmectomy, and secondary preventive pharmacotherapy, whereas patients with NICM may have better response to beta-blockade.11
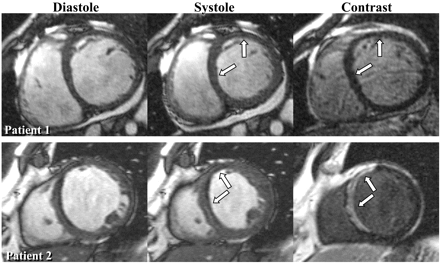
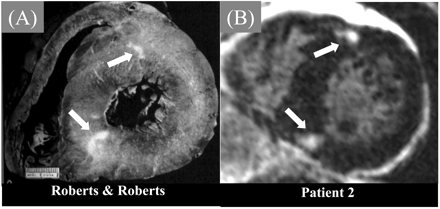
Wu et al.12 were the first to report that DE-CMR may hold promise in differentiating ischaemic from NICM. In their study, nearly all patients with ischaemic heart disease and prior myocardial infarction had myocardial hyperenhancement, whereas none of the patients with idiopathic DCM or the normal volunteers had hyperenhancement. In a more recent study, Bello et al.13 evaluated a cohort of patients with congestive heart failure and documented systolic dysfunction. In this study, the level of systolic dysfunction was quite severe (mean EF was 26±11%), hyperenhancement was found in 100% of patients with ICM, but in only 12% with idiopathic DCM. From these data we can conclude that myocardial hyperenhancement consistent with gross myocardial scarring is virtually always present in patients with chronic ICM, whereas it is relatively rare in patients with idiopathic DCM (Figure 2). This conclusion is consistent with previous pathology studies. Schuster and Bulkley14 demonstrated that virtually all patients with congestive heart failure and significant CAD have gross myocardial scarring at autopsy, even in those without clinical history of myocardial infarction, angina, or Q-waves. Conversely, Roberts et al.15 found visible scars at necropsy in only 14% of patients with idiopathic DCM.
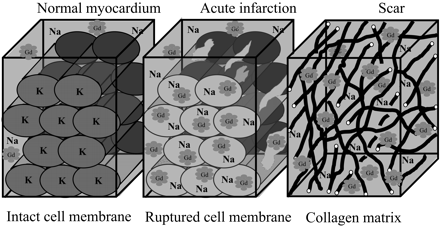
The mechanism of myocardial hyperenhancement in the setting of infarction is demonstrated in Figure 3. The mechanism is based on two simple facts. First, gadolinium chelates are extra cellular contrast agents that are inert and cannot cross myocyte cell membranes.16 Secondly, in normal myocardium, myocytes are densely packed and thus myocyte intracellular space forms the majority (∼85%) of the volume.17 Conceptually, it then follows that the volume of distribution of gadolinium in a hypothetical voxel of normal myocardium is small (Figure 3), and the overall number of gadolinium molecules is low. In the setting of acute infarction, there is myocyte membrane rupture, which allows additional gadolinium to diffuse into what was previously intracellular space. This in turn results in increased gadolinium concentration and therefore hyperenhancement. In the setting of chronic infarction, myocytes have been replaced with collagenous scar. In this situation, the interstitial space is expanded18 which again leads to increased gadolinium concentration and therefore hyperenhancement. Given the mechanism of hyperenhancement in the setting of prior myocardial infarction, along with sensitivity of DE-CMR to detect even small subendocardial infarcts, the finding that nearly 100% of patients with chronic ICM have hyperenhancement, is consistent with the pathophysiology of this disease.
Recently, McCrohon et al.19 performed DE-CMR in 90 patients with ischaemic (n=27) or idiopathic DCM (n=63) on the basis of coronary angiography. Although all patients with ICM had myocardial hyperenhancement, 41% of patients with idiopathic DCM also had hyperenhancement. Although the prevalence of hyperenhancement in patients with idiopathic DCM appears to be significantly higher than that reported by Wu et al.12 and Bello et al.,13 it is important to note that there were differences on how hyperenhancement was defined in these studies. For instance, in the earlier studies, only hyperenhancement patterns consistent with regions of prior myocardial infarction were included in the analysis.
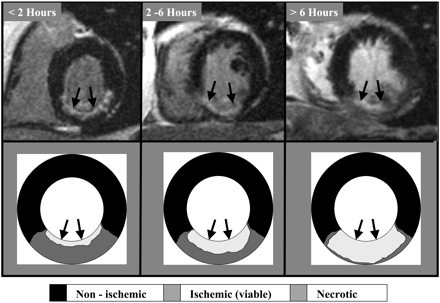
The typical pattern of hyperenhancement that occurs in patients with prior myocardial infarction and thus in those with ICM can be explained by the pathophysiology of ischaemia (Figure 4). Following coronary occlusion, myocardial contractile function falls almost immediately throughout the region of ischaemia. However, little or no cellular necrosis is found until ∼15 min after occlusion. From this point in time onwards, a ‘wave front’ of necrosis begins in the subendocardium and grows towards the epicardium over the next few hours. During this period the region of ischaemia remains the same size but the infarcted region within the ischaemic zone increases continuously towards a transmural infarction. Therefore, hyperenhancement can be classified as ‘ischaemic-type’ or ‘non-ischaemic type’, and the former should always involve the subendocardium (i.e. subendocardial or transmural) and be located in a region, which is consistent with the perfusion territory of an epicardial coronary artery. Using this classification process, McCrohon et al.19 found that 13% of patients with idiopathic ICM had ischaemic-type hyperenhancement, whereas 28% had non-ischaemic type. These findings are consistent with those of Bello et al.,13 though the rate of ischaemic-type hyperenhancement was quite low in both studies (13 vs.12%).
The clinical interpretation of ischaemic-type hyperenhancement in patients with idiopathic DCM is unclear. Certainly the occurrence of recanalization after an occlusive coronary event or embolization from minimally stenotic but unstable plaque is well documented.20,21 Therefore, McCrohon et al.19 believed that these patients were incorrectly diagnosed by coronary angiography as patients with idiopathic DCM.
In the study by McCrohon et al.,19 patchy or linear striae of hyperenhancement limited to the mid-myocardium of the ventricular wall were considered as non-ischaemic type enhancement. An example of such hyperenhancement is demonstrated in Figure 5. In our experience, linear midwall hyperenhancement is usually found in the interventricular septum. It is observed more frequently in patients with long-standing LV dysfunction and in those with possible prior history of myocarditis.
Contrast CMR in the setting of NICM
The studies by Wu et al.,12 Bello et al.,13 and McCrohon et al.19 involved only patients with ICM or those with idiopathic DCM. Patients with HCM, myocarditis, or infiltrative disorders were excluded. In this section, we will demonstrate that hyperenhancement can occur in these other forms of NICM and describe how the pattern of hyperenhancement can be used to differentiate between the various aetiologies.
HCM
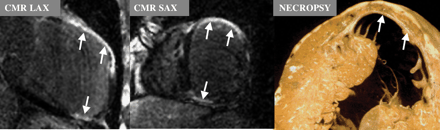
Choudhury et al.22 performed DE-CMR in 21 patients previously diagnosed with HCM. In these patients the maximum end-diastolic wall thickness averaged 25±8 mm, and they had preserved EF (70±11%). Hyperenhancement was found in the majority (81%) of patients, although the extent of hyperenhancement was usually quite limited (8±9% of LV mass). The pattern of hyperenhancement was peculiar, occurring only in hypertrophied regions, predominantly involving the middle third of the ventricular wall in a patchy, multi-focal distribution. Interestingly, all patients with hyperenhancement had involvement at the junctions of the interventricular septum and the right ventricular(RV) free wall (Figure 6). These findings have been confirmed by Moon et al.23, who described a similar prevalence and pattern of hyperenhancement in 53 patients with HCM, and are also consistent with previous studies.24
Myocarditis
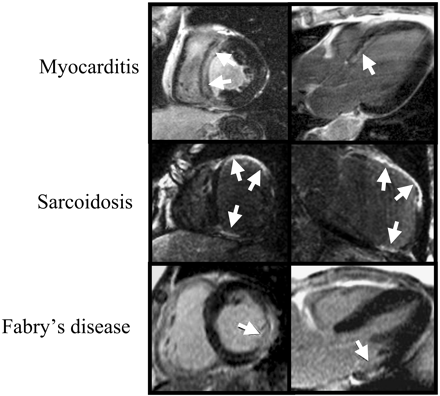
The study by Friedrich et al.25 was the first to systematically evaluate myocardial hyperenhancement in patients with myocarditis. In this study, the CMR technique was a non-breath-hold T1-weighted spin-echo pulse sequence.25 The authors showed that hyperenhancement in patients with myocarditis evolves during the first 2 weeks after the onset of symptoms and concluded that CMR may hold promise for diagnosing inflammatory heart disease.25 However, regions of hyperenhancement had image intensities that were on average only 40–50% higher than normal regions. Given that newer DE-CMR techniques provide an order of magnitude improvement in contrast-to-noise ratio for detecting necrotic myocardium compared with older spin-echo techniques,6 we performed a study in 32 patients with suspected myocarditis using these newer techniques.26 We found that image intensities in regions of hyperenhancement were on average 400% higher than in normal regions, confirming that hyperenhancement is a frequent finding in clinically suspected myocarditis, and that it is associated with active myocarditis defined by histopathology.26 In addition, we observed that hyperenhancement in the setting of myocarditis has a ‘non-ischaemic’ pattern, typically affecting the epicardial quartile of the ventricular wall, may decrease during healing and can be almost invisible after recovery (Figure 7).
Chagas disease
Chagas is an inflammatory disease caused by the parasitic protozoan Trypanosoma cruzi. Although most patients survive the acute phase of the disease and remain asymptomatic for many years, ∼20% of patients eventually have chronic heart failure. Recent pilot data suggest that DE-CMR may be capable of detecting early cardiac involvement in the setting of Chagas disease before the onset of symptom.27 In these patients, hyperenhancement is present in a non-ischaemic type pattern. Frequently, the epicardial or midwall portion of the inferolateral wall is involved (Figure 8). However, other regions such as the apex may also be affected and in some patients the pattern of hyperenhancement may resemble that seen in patients with CAD.
Sarcoid
In patients with sarcoidosis, cardiac involvement is found in 20–30% at autopsy and likely plays a role in sudden death. However, cardiac involvement is clinically evident in <5% of patients as current diagnostic tools are insensitive. Several authors have emphasized the occurrence of MR abnormalities (especially on T2-weighted images) in patients with ongoing systemic sarcoidosis.28,29 Recently Patel et al.30 performed DE-CMR in 58 consecutive patients with biopsy proven systemic sarcoidosis. In this pilot study, a two-fold higher rate of cardiac involvement was diagnosed by CMR than by Japanese Ministry of Health clinical criteria. Frequently, hyperenhancement was found isolated to the mid-myocardial wall or epicardium, indicative of a non-ischaemic type pattern. However, subendocardial or transmural hyperenhancement was also observed, mimicking the pattern of myocardial infarction (Figure 9).
Anderson–Fabry disease
Fabry disease is an X-linked recessive storage disorder with variable phenotype characterized by the accumulation of glycosphingolipid in various tissues. Unlike patients with the classical systemic Fabry disease entity, who present with multiple organ involvement, patients with a cardiac variant of Fabry are characterized mainly by myocardial hypertrophy. In these patients, the cardiac phenotype is similar to that seen in HCM, and the diagnosis may be difficult. This is of clinical importance, as recent data suggest that the prevalence of treatable Fabry disease in a typical HCM referral population may be as high as 5%.31 Although the pattern of ventricular hypertrophy caused by Fabry disease may be similar to that seen in HCM, the pattern of hyperenhancement seen on DE-CMR appears to be different. Moon et al.32 recently reported that 50% of patients with genetically confirmed Fabry disease have myocardial hyperenhancement. In these patients, hyperenhancement was most frequently observed in the basal inferolateral wall, although the subendocardial portion was usually spared. However, for the final diagnosis of Fabry disease additional testing such as biopsy or genotyping is obviously needed.
Amyloid
Cardiac amyloidosis is a common cause for restrictive ventricular dysfunction and can cause cardiac death shortly after the onset of heart failure. LV hyperenhancement in cardiac amyloidosis is usually diffuse.33 Although the subendocardium may be involved, such as that seen in ischaemic heart disease, the distribution often is global and does not match any specific coronary artery perfusion territory (Figure 10).33 From a technical point of view, this diffuse involvement may make the setting of the parameters for DE-CMR difficult. For example, it may be difficult to determine the optimal inversion time that will null normal myocardium, as it may be unclear which myocardial areas are normal. In this situation, a helpful approach may be to acquire multiple images of the same view using different inversion times (e.g. TI-SCOUT, Siemens-Medical-Systems, Erlangen, Germany). We have found empirically that if a large portion of LV myocardium (i.e.>50%) goes through the null point earlier than the LV cavity and this region is not in an obvious coronary artery distribution territory the diagnosis of cardiac amyloidosis is highly likely. Unfortunately, this method requires that a substantial portion of LV myocardium is involved. The sensitivity and specificity of DE-CMR for identifying mild forms of cardiac amyloidosis are currently unknown.
Pathophysiological basis of hyperenhancement in NICM
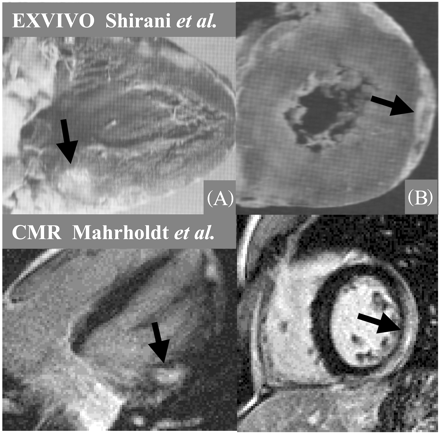
Currently there are two lines of evidence indicating that areas of gadolinium hyperenhancement represent regions of myocardial necrosis or scarring. First, the locations and patterns of hyperenhancement seen in vivo in patients with various forms of NICM appear to match the locations and patterns of scarring seen in gross pathology specimens of necropsy studies. Scarring in the setting of HCM, for example, does not relate to any epicardial coronary artery distribution area, is most prominent at the junctions of the interventricular septum as well as the RV free walls (along with myocardial disarray)34–39 and exactly matches the pattern of hyperenhancement22 (Figure 11). Secondly, there is now data, although limited, comparing in vivo DE-CMR findings in a HCM patient with gross pathology findings 49 days later following after cardiac transplantation. In this study, there was a direct relationship between the presence of hyperenhancement on DE-CMR and the presence of replacement scarring on pathology.40
Replacement scarring, however, only represents one type of myocardial fibrosis occurring in HCM. Another unique entity characteristic for myocardium exhibiting myocardial fibre disarray is referred to by the term ‘plexiform fibrosis’.41 Would this form of fibrosis also lead to focal hyperenhancement following the administration of gadolinium? This is unlikely for two reasons. First, DE-CMR is sensitive to regional differences in gadolinium accumulation rather than to an overall increase because the technique depends on the ability to ‘null’ signal from ‘remote’ (presumably normal) myocardium. Therefore, cardiac disorders that lead to focal regions of scarring will enhance, whereas disorders that lead to global changes such as diffuse interstitial fibrosis will not. Secondly, it should be noted that the voxel resolution of DE-CMR is ∼1.9 mm×1.4 mm×6 mm. Although the pathologic analogue of a scar visible on CMR comprises several voxels, it will also be visible to the naked eye and is therefore not likely to represent microscopic scarring. Thus, DE-CMR depicts areas of scarring in vivo that previously could only be detected at autopsy but it is not to delineate diffuse reticular interstitial scar tissue.
This concept of in vivo DE-CMR detection of macroscopic scarring is not only consistent with the findings of Wu et al.,12 Bello et al.,13 and McCrohon et al.19 but it also explains the pattern of hyperenhancement in the setting of sarcoidosis30 as displayed in Figure 9. Note that the distribution of hyperenhancement exactly matches areas of thinning caused by sarcoid scarring. Moreover, autopsy demonstrates disseminated myocardial necrosis in myocarditis42,43 and consequently, the pattern of hyperenhancement found in myocarditis is also remarkably similar to the pattern of necrosis seen post-mortem (Figure 12).
Overall imaging approach to the assessment of CMs
In the clinical routine most patients are first evaluated by echocardiography. The echocardiographic determination of the exact aetiology, however, is limited to the assessment of wall motion abnormalities, ventricular dilatation, or patterns of hypertrophy. Thus, we propose to additionally use DE-CMR to evaluate the presence or absence of hyperenhancement. If no hyperenhancement is present in the setting of a diffusely impaired ventricle, the most likely diagnosis is idiopathic DCM, although depending on the history, alcoholic or post-partum CM is also a possibility.
If hyperenhancement is present, the pattern of hyperenhancement needs to be examined. In the setting of subendocardial or transmural hyperenhancement, this pattern is consistent with the presence of CAD (Figures 4 and 13), and ICM is the most likely diagnosis. However, if the pattern of hyperenhancement is not of the ischaemic-type, NICM is likely to be present. Distinctive midwall striae (Figure 5) can be seen in up to 30% of idiopathic DCM patients19 and may correspond to the remnants of previous myocarditis. Focal or diffuse hyperenhancement in hypertrophied regions and involvement at the junctions of the interventricular septum and the RV free wall indicates HCM21 (Figures 6,11, and 13).
Infiltrative disorders such as sarcoidosis or Fabry disease are characterized by intramural or subepicardial areas of hyperenhancement, which are not related to any coronary perfusion territory (Figure 13). In the setting of myocarditis, hyperenhancement also frequently involves the subepicardium (Figure 7). As a unique hallmark of myocarditis, hyperenhancement will decrease after healing as shown in the bottom panel of Figure 7. However, larger areas of scarring caused by myocarditis may still be visible after healing, often resembling intramural ‘striae like’ structures as displayed in Figures 13 and 14.
An evaluation of this approach was recently given by Patel et al.44 in 234 consecutive patients presenting for work up of unclear CM (EF≤30%). One-hundred and seventy-two patients underwent catheterization and CMR. A non-ischaemic pattern of contrast enhancement was defined as isolated mid-myocardial or epicardial hyperenhancement. Although, simply the presence of hyperenhancement was sensitive (94%) for the diagnosis of ICM, the specificity (60%) was relatively poor. When that pattern of hyperenhancement was taken into account (ischaemic or non-ischaemic type) the sensitivity remained high (92%), whereas there was a significant improvement in specificity (93%). Although this is a single pilot study, these data indicate the potential clinical utility of this approach.
Potential complexities for clinical image interpretation
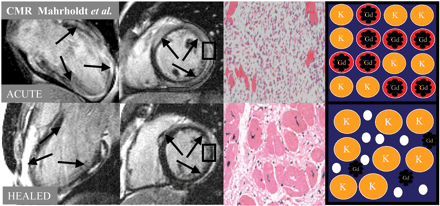
Several issues may complicate the clinical interpretation of DE-CMR imaging. One such issue is that in the setting of myocarditis, hyperenhancement can decrease significantly or even disappear during healing25,26 (Figure 14). According to histopathology there is a fundamental difference between necrosis due to myocarditis, and necrosis in the setting of acute infarction. Areas of necrotic tissue within a myocarditic focus are usually smaller than in infarcts and islands of necrotic cells are dispersed in a ‘cougar like pattern’ throughout the inflammatory focus.45–47 Just as in infarcts, acute necrosis in the setting of myocarditis is characterized by ruptured sarcolemmal membranes and surrounding interstitial oedema, allowing the contrast agent to accumulate in the interstitium, and to diffuse into the intracellular space. Thus, the general mechanism of hyperenhancement in myocarditis is the same as in infarction. During healing some amount of interstitial oedema persists and necrotic myocytes are slowly replaced by fibrous tissue, comparable with small chronic infarcts. Thus, hyperenhancement will remain present during this phase of the disease. However, when healing is completed, oedema has resolved while scars shrink and remodel over time. Consequently, voxels may now contain so many previously bordering surviving myocytes, that hyperenhancement in these voxels may no longer be visible, resulting the area of hyperenhancement to decrease significantly. This effect is likely magnified by the patchy distribution of scars in the setting of myocarditis and the fact that interstitial oedema only occurs in areas of myocardial necrosis.17 Thus, all visible hyperenhancement may disappear in some cases after healing, just leaving minimal diffuse signal enhancement as reported by Friedrich et al.25 and Wagner et al.48
This concept is illustrated in Figure 14. Interestingly the biopsy sample taken in the acute setting from a patient exhibiting diffuse hyperenhancement in the entire LV shows significantly larger interstitial space (due to necrosis and oedema) than the sample taken during healing when much of the hyperenhancement has disappeared. Obviously, this pair of biopsy samples is no proof of this concept because of the possible sampling error. However, in this special case hyperenhancement was present in the entire ventricle during the acute phase and decreased significantly nearly everywhere after healing. Thus, the biopsy samples might very well have been taken from an area that was hyperenhanced in the acute, and is not hyperenhanced in the healed setting.
Another issue complicating clinical image interpretation is the fact that hyperenhancement may be present in an ischaemic-type pattern but no CAD can be found by angiography. There are several possible explanations for this scenario. One is that, in fact, there is epicardial CAD present, but was missed by coronary angiography for some reason. However, in the setting of global LV dysfunction this explanation seems not very likely. In addition, NICMs may rarely mimic the pattern of infarct related hyperenhancement as shown in Figure 15.
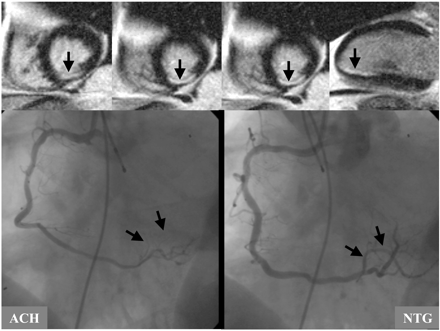
A third issue complicating clinical image interpretation may be a scenario in which the patient has had myocardial infarction but without any epicardial CAD. The occurrence of spontaneous recanalization after an occlusive coronary event or embolization from minimally stenotic but unstable plaque is possible and underlying mechanisms are well documented.20,21 In addition to that, other mechanisms can cause myocardial infarction without the presence of epicardial CAD. The patient displayed in Figure 16 suffered inferior myocardial infarction resulting from coronary spasm as indicated by angiography. During intracoronary application of acetylcholine (ACH) severe spasm in the area of the distal right coronary artery (RCA) can be seen. After intracoronary administration of nitro-glycerine (NTG) the spasm and the clinical symptoms disappeared.
Clinical implications
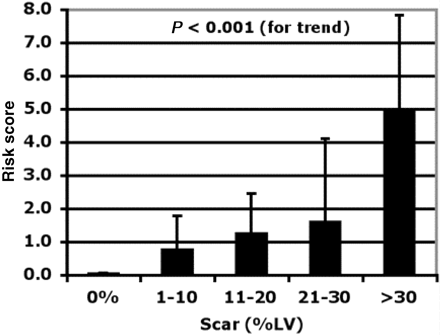
Hyperenhancement assessed using state of the art DE-CMR techniques is capable of differentiating between ischaemic and non-ischaemic forms of CM in patients with large, diffusely hypocontractile ventricles. Moreover, in patients with other forms of CM, CMR is often helpful to determine a specific aetiology. In addition, hyperenhancement cannot only be used for diagnostic purposes, but also provides information on the exact amount of irreversible myocardial damage, which is likely to be relevant for the patients' prognosis. Three lines of evidence point to the prognostic relevance of hyperenhancement in patients with CMs. (i) A certain amount of irreversible myocardial damage may cause adverse ventricular remodelling as described by Moon et al.23 showing that in the setting of HCM the extent of hyperenhancement is associated with ventricular dilation and clinical symptoms.23,24 (ii) Myocardial areas affected by irreversible damage will not respond to heart failure drug therapy as reported by Bello et al.13 and (iii) Irreversible myocardial damage may trigger arrhythmic events causing sudden cardiac death, which is consistent with several reports of a progressive stepwise relationship between the amount of scarring and the clinical risk for sudden death in the setting of HCM 23,49 (Figure 17). However, despite the obvious diagnostic utility of DE-CMR its independent prognostic value will need to be determined.
Supplementary material
Supplementary material is available at European Heart Journal online.
Acknowledgements
This work was supported in part by a grant from the Robert-Bosch-Foundation (HM) and by R01-HL64726 (R.K.)
Reprints are available from RJK. E-mail address: Raymond.Kim@dcmrc.mc.duke.edu
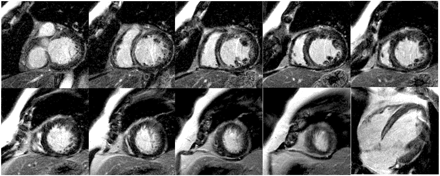
Figure 1 DE-CMR views before and after coronary stenting. Note new discrete regions of hyperenhancement in the inferior wall representing areas of procedure related micro scarring. Adapted with permission from Ricciardi et al.8
Figure 2 The left columns display cine-CMR short axis images in two patients presenting with heart failure. In both patients the LV is enlarged and there is regional dysfunction primarily in the anteroseptal wall. The corresponding DE-CMR views demonstrate no hyperenhancement in the patient with NICM, whereas there is transmural hyperenhancement in the anteroseptal wall consistent with a prior myocardial infarction in the patient with ischaemic heart disease (patient 2). Reprinted with permission from Bello et al.13 Full motion cine movies can be viewed at http://dcmrc.mc.duke.edu/bb4/index.html.
Figure 3 Mechanisms of contrast enhancement in myocardial infarction. See text for details. Adapted with permission from Kim RJ et al.50 (See Supplementary material online for a colour version of this figure.)
Figure 4 The typical pattern of ischaemic heart disease can be explained by the pathophysiology of ischaemia. Little or no cellular necrosis is found until about 15 min after occlusion. After 15 min, a ‘wavefront’ of necrosis begins subendocardial and grows towards the epicardium over the next few hours. During this period the infarcted region within the ischaemic zone increases continuously towards a transmural infarction.
Figure 5 DE-CMR images in a patient presenting with idiopathic DCM. Note the intramural contrast enhancement in the septum (midwall striae) that can be found in up to 30% of idiopathic DCM cases.
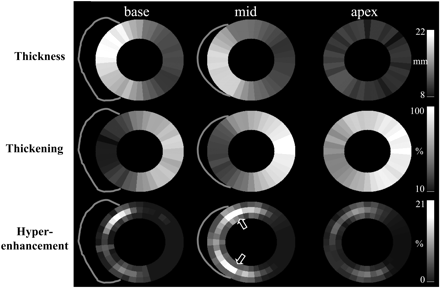
Figure 6 Spatial distribution of the mean values for segmental wall thickness, wall thickening and scar extent (hyperenhancement) represented as grey-scale maps in patients with HCM. Note that the thicker walls have the least thickening. Scarring predominantly involves the midwall myocardium at the junctions of the interventricular septum and the RV free wall. Reprinted with permission from Choudhury et al.22
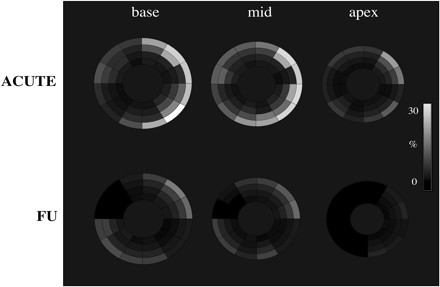
Figure 7 Spatial distribution of the mean values for segmental extent of hyperenhancement represented as grey-scale maps in patients with acute myocarditis and at follow-up. Reprinted with permission from Mahrholdt et al.26
Figure 8 Hyperenhancement in the setting of Chagas disease. In these patients hyperenhancement is often present in a non-ischaemic type pattern. Frequently, the epicardial or midwall portion of the inferolateral wall is involved. Images by Dr Carlos Rochitte, Sao-Paulo, Brasil.
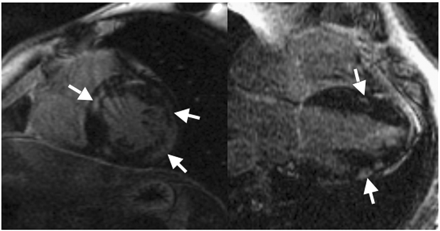
Figure 9 Comparison between in vivo DE-CMR in one patient with a pathology image from a different patient with cardiac sarcoid studied at necropsy. Note that the distribution of hyperenhancement matches areas of thinning caused by sarcoid infiltration.
Figure 10 Comparison between in vivo DE-CMR in one patient with pathology and corresponding histology images (Congo Red stained) from a different patient with cardiac amyloid. Note that the distribution of hyperenhancement matches the diffuse subendocardial amyloid (red stained area on left side of histologic image).
Figure 11 Comparison between in vivo DE-CMR in patients (B) with reproduced pathology figures from a different patient with HCM studied at necropsy (A). Arrows point to regions of hyperenhancement on DE-CMR or regions of scar on pathology. The locations of hyperenhancement in the living patient (B) are remarkably similar to the locations of scarring in the reproduced pathology figures (A). Panel B is modified with permission from Choudhury et al.22 and panel A is reproduced with permission from Roberts and Roberts37 and Basso et al.34
Figure 12 Comparison between in vivo DE-CMR in one patient (bottom panel) with reproduced pathology figures from two different patients. Arrows point to regions of myocarditic infiltration on DE-CMR imaging and on pathology. Note the close correlation between CMR and necropsy. The upper panel is reproduced with permission from Shirani et al.43 and the bottom panel from Mahrholdt et al.26
Figure 13 Hyperenhancement patterns that one may encounter in clinical practice. If hyperenhancement is present, the endocardium should be involved in patients with ischaemic disease. Isolated midwall or epicardial hyperenhancement strongly suggests a ‘non-ischaemic’ aetiology. Reprinted by permission from Shah et al.51 (see Supplementary material online for a colour version of this figure).
Figure 14 The left columns display CMR images of a patient with myocarditic infiltrations. Note that the hyperenhancement decreases significantly during healing. Comparing CMR guided biopsy samples acute vs. healed (third column) shows a significant decrease of interstitial/extra-cellular space. This shrinkage of interstitial/extra-cellular space during healing in combination with the cougar like pattern of myocarditic lesions explains why hyperenhancement may fade over time in myocarditis. This mechanism is also visualized by the cartoons in the right column (also compare with Figure 3). The left two panels are reproduced with permission from Mahrholdt et al.26
Figure 15 This figure displays how different NICMs can mimic the contrast pattern of myocardial infarction. The patient in the upper row has biopsy proven HHV6 myocarditis and no CAD by angiography. Cardiac sarcoidosis can cause transmural infiltrations resulting in wall thinning just as myocardial infarction. However, the area of hyperenhancement does not fit any coronary perfusion area. The infiltrations of Fabry disease are normally intramural, except for few cases were also the subendocardium can be affected. Bottom panel modified with permission from Moon et al.32
Figure 16 The patient displayed in this figure suffered inferior myocardial infarction resulting from coronary spasm. During intracoronary application of ACH severe spasm in the area of the distal RCA can be seen, causing the patients typical angina for the duration of the test. After intracoronary administration of NTG both the spasm and the clinical symptoms disappeared.
Figure 17 Bar graph visualizing a progressive stepwise relationship between the extent of myocardial scarring assessed by DE-CMR and the clinical risk of sudden cardiac death in HCM. Reprinted with permission Mahrholdt et al.49
Morphological and functional abnormalities associated with cardiomyopathy
| . | DCM . | HCM . | RCM . |
|---|---|---|---|
| Morphology | |||
| LV and RV size | +++ | − | − |
| Hypertrophy | −/+ | +++ | + |
| Atrial dilatation | ++ | + | ++ |
| Pleural effusion | + | −/+ | + |
| Pericardial effusion | + | − | + |
| Ventricular thrombus | + | − | − |
| SVC/IVC dilatation | + | − | +++ |
| Myocardial function | |||
| Global dysfunction | +++ | + | + |
| Segmental dysfunction | + | +++ | + |
| Diastolic dysfunction | + | ++ | +++ |
| Valvular function | |||
| Mitral regurgitation | + | ++ | + |
| Tricuspid regurgitation | + | − | + |
| . | DCM . | HCM . | RCM . |
|---|---|---|---|
| Morphology | |||
| LV and RV size | +++ | − | − |
| Hypertrophy | −/+ | +++ | + |
| Atrial dilatation | ++ | + | ++ |
| Pleural effusion | + | −/+ | + |
| Pericardial effusion | + | − | + |
| Ventricular thrombus | + | − | − |
| SVC/IVC dilatation | + | − | +++ |
| Myocardial function | |||
| Global dysfunction | +++ | + | + |
| Segmental dysfunction | + | +++ | + |
| Diastolic dysfunction | + | ++ | +++ |
| Valvular function | |||
| Mitral regurgitation | + | ++ | + |
| Tricuspid regurgitation | + | − | + |
Morphological and functional abnormalities associated with cardiomyopathy
| . | DCM . | HCM . | RCM . |
|---|---|---|---|
| Morphology | |||
| LV and RV size | +++ | − | − |
| Hypertrophy | −/+ | +++ | + |
| Atrial dilatation | ++ | + | ++ |
| Pleural effusion | + | −/+ | + |
| Pericardial effusion | + | − | + |
| Ventricular thrombus | + | − | − |
| SVC/IVC dilatation | + | − | +++ |
| Myocardial function | |||
| Global dysfunction | +++ | + | + |
| Segmental dysfunction | + | +++ | + |
| Diastolic dysfunction | + | ++ | +++ |
| Valvular function | |||
| Mitral regurgitation | + | ++ | + |
| Tricuspid regurgitation | + | − | + |
| . | DCM . | HCM . | RCM . |
|---|---|---|---|
| Morphology | |||
| LV and RV size | +++ | − | − |
| Hypertrophy | −/+ | +++ | + |
| Atrial dilatation | ++ | + | ++ |
| Pleural effusion | + | −/+ | + |
| Pericardial effusion | + | − | + |
| Ventricular thrombus | + | − | − |
| SVC/IVC dilatation | + | − | +++ |
| Myocardial function | |||
| Global dysfunction | +++ | + | + |
| Segmental dysfunction | + | +++ | + |
| Diastolic dysfunction | + | ++ | +++ |
| Valvular function | |||
| Mitral regurgitation | + | ++ | + |
| Tricuspid regurgitation | + | − | + |
References
Richardson P, McKenna W, Bristow M. Report of the 1995 World Health Organization/International Society and Federation of Cardiology Task Force on the definition and classification of cardiomyopathies.
Bender JR. Idiopathic dilated cardiomyopathy. An immunologic, genetic or infectious disease, or all of the above?
Spirito P, Seidman CE, McKenna WJ. The management of hypertrophic cardiomyopathy. (Review).
Felker GM, Thompson RE, Hare JM. Underlying causes and long-term survival in patients with initially unexplained cardiomyopathy.
Kubo N, Morimoto S, Hiramitsu S. Feasibility of diagnosing chronic myocarditis by endomyocardial biopsy.
Simonetti OP, Kim RJ, Fieno DS. An improved MR imaging technique for the visualization of myocardial infarction.
Mahrholdt H, Wagner A, Judd RM. Assessment of myocardial viability by cardiovascular magnetic resonance imaging.
Ricciardi MJ, Wu E, Davidson CJ. Visualization of discrete microinfarction after percutaneous coronary intervention associated with mild creatine kinase-MB elevation.
Wagner A, Mahrholdt H, Holly T. Contrast-enhanced MRI and routine single photon emission computed tomography (SPECT) perfusion imaging for detection of subendocardial myocardial infarcts: an imaging study.
Felker GM, Shaw LK, O'Connor CM. A standardized definition of ischemic cardiomyopathy for use in clinical research.
Bristow MR, Gilbert EM, Abraham WT. Carvedilol produces dose-related improvements in left ventricular function and survival in subjects with chronic heart failure. MOCHA Investigators.
Wu E, Judd RM, Vargas J. Visualisation of presence, location, and transmural extent of healed Q-wave and non-Q-wave myocardial infarction.
Bello D, Shah DJ, Farah GM. Gadolinium cardiovascular magnetic resonance predicts reversible myocardial dysfunction and remodeling in patients with heart failure undergoing beta-blocker therapy.
Schuster EH, Bulkley BH. Ischemic cardiomyopathy: a clinicopathologic study of fourteen patients.
Roberts WC, Siegel RJ, McManus BM. Idiopathic dilated cardiomyopathy: analysis of 152 necropsy patients.
Koenig SH, Spiller M, Brown RD III. Relaxation of water protons in the intra- and extracellular regions of blood containing Gd(DTPA).
Kim RJ, Judd RM, Chen EL. Relationship of elevated 23Na magnetic resonance image intensity to infarct size after acute reperfused myocardial infarction.
Rehwald WG, Fieno DS, Chen EL. Myocardial magnetic resonance imaging contrast agent concentrations after reversible and irreversible ischemic injury.
McCrohon JA, Moon JC, Prasad SK. Differentiation of heart failure related to dilated cardiomyopathy and coronary artery disease using gadolinium-enhanced cardiovascular magnetic resonance.
Topol EJ, Yadav JS. Recognition of the importance of embolization in atherosclerotic vascular disease.
Arroyo LH, Lee RT. Mechanisms of plaque rupture: mechanical and biologic interactions.
Choudhury L, Mahrholdt H, Wagner A. Myocardial scarring in minimally symptomatic patients with hypertrophic cardiomyopathy.
Moon JCC, McKenna WJ, McCrohon JA. Toward clinical risk assessment in hypertrophic cardiomyopathy with gadolinium cardiovascular magnetic resonance.
Wilson JM, Villareal RP, Hariharan R. Magnetic resonance imaging of myocardial fibrosis in hypertrophic cardiomyopathy.
Friedrich MG, Strohm O, Schulz-Menger J. Contrast media-enhanced magnetic resonance imaging visualizes myocardial changes in the course of viral myocarditis.
Mahrholdt H, Goedecke C, Wagner A. Cardiovascular magnetic resonance assessment of human myocarditis; a comparison to histology and molecular pathology.
Rochitte CE, Olivera PF, Joalbo M. Chagas disease is characterized by specific pattern and location of myocardial delayed enhanced MRI. (Abstract).
Kurashima K, Shimizu H, Ogawa H. MR and CT in the evaluation of sarcoid myopathy.
Vignaux O, Dhote R, Duboc D. Detection of myocardial involvement in patients with sarcoidosis applying T2-weighted, contrast enhanced, and cine magnetic resonance imaging: initial results of a prospective study.
Patel MR, Cawely PJ, Heitner JF. Improved diagnostic sensitivity of contrast enhanced cardiac MRI for cardiac sarcoidosis. (Abstract).
Sachdev B, Takenaka T, Teraguchi H. Prevalence of Anderson-Fabry disease in male patients with late onset hypertrophic cardiomyopathy.
Moon JC, Sachdev B, Elkington AG. Gadolinium enhanced cardiovascular magnetic resonance in Anderson-Fabry disease. Evidence for a disease specific abnormality of the myocardial interstitium.
Basso C, Thiene G, Corrado D. Hypertrophic cardiomyopathy and sudden death in the young: pathologic evidence of myocardial ischemia.
Maron BJ, Epstein SE, Roberts WC. Hypertrophic cardiomyopathy and transmural myocardial infarction without significant atherosclerosis of the extramural coronary arteries.
Maron BJ, Wolfson JK, Epstein SE. Intramural (“small vessel”) coronary artery disease in hypertrophic cardiomyopathy.
Roberts CS, Roberts WC. Morphologic features. In: Zipes DP, Rowlands DJ, eds.
Varnava AM, Elliott PM, Sharma S. Hypertrophic cardiomyopathy: the interrelation of disarray, fibrosis, and small vessel disease.
Kuribayashi T, Roberts WC. Myocardial disarray at junction of ventricular septum and left and right ventricular free walls in hypertrophic cardiomyopathy.
Moon JC, Reed E, Sheppard MN. The histologic basis of late gadolinium enhancement cardiovascular magnetic resonance in hypertrophic cardiomyopathy.
Anderson KR, Sutton MG, Lie JT. Histopathological types of cardiac fibrosis in myocardial disease.
Kawai C. From myocarditis to cardiomyopathy: mechanisms of inflammation and cell death: learning from the past for the future.
Shirani J, Freant LJ, Roberts WC. Gross and semiquantitative histologic findings in mononuclear cell myocarditis causing sudden death, and implications for endomyocardial biopsy.
Patel MR, Heitner JF, Klem I. Presence and pattern of scar on delayed-enhanced MRI differentiates ischemic from non-ischemic cardiomyopathy. (Abstract).
Aretz HT, Billingham ME, Edwards WD. Myocarditis: a histopathologic definition and classification.
Hauck AJ, Kearney DL, Edwards WD. Evaluation of postmortem endomyocardial biopsy specimens from 38 patients with lymphocytic myocarditis: implications for role of sampling error.
Klingel K, Stephan S, Sauter M. Pathogenesis of murine enterovirus myocarditis: virus dissemination and immune cell targets.
Wagner A, Schulz-Menger J, Dietz R. Long-term follow-up of patients with acute myocarditis by magnetic resonance imaging.
H. Mahrholdt, L Choudhury, A. Wagner. Relation of myocardial scarring to clinical risk factors for sudden cardiac death in hypertrophic cardiomyopathy. (Abstract)
Kim RJ Choi KM, Judd RM. Assessment of myocardial viability by contrast enhancement. In: Higgins CB, de Roos A, eds.