-
PDF
- Split View
-
Views
-
Cite
Cite
Dennis C. Sgroi, Erin Carney, Elizabeth Zarrella, Lauren Steffel, Shemeica N. Binns, Dianne M. Finkelstein, Jackie Szymonifka, Atul K. Bhan, Lois E. Shepherd, Yi Zhang, Catherine A. Schnabel, Mark G. Erlander, James N. Ingle, Peggy Porter, Hyman B. Muss, Katherine I. Pritchard, Dongsheng Tu, David L. Rimm, Paul E. Goss, Prediction of Late Disease Recurrence and Extended Adjuvant Letrozole Benefit by the HOXB13/IL17BR Biomarker, JNCI: Journal of the National Cancer Institute, Volume 105, Issue 14, 17 July 2013, Pages 1036–1042, https://doi.org/10.1093/jnci/djt146
Close - Share Icon Share
Abstract
Biomarkers to optimize extended adjuvant endocrine therapy for women with estrogen receptor (ER)–positive breast cancer are limited. The HOXB13/IL17BR (H/I) biomarker predicts recurrence risk in ER-positive, lymph node–negative breast cancer patients. H/I was evaluated in MA.17 trial for prognostic performance for late recurrence and treatment benefit from extended adjuvant letrozole.
A prospective–retrospective, nested case-control design of 83 recurrences matched to 166 nonrecurrences from letrozole- and placebo-treated patients within MA.17 was conducted. Expression of H/I within primary tumors was determined by reverse-transcription polymerase chain reaction with a prespecified cutpoint. The predictive ability of H/I for ascertaining benefit from letrozole was determined using multivariable conditional logistic regression including standard clinicopathological factors as covariates. All statistical tests were two-sided.
High H/I was statistically significantly associated with a decrease in late recurrence in patients receiving extended letrozole therapy (odds ratio [OR] = 0.35; 95% confidence interval [CI] = 0.16 to 0.75; P = .007). In an adjusted model with standard clinicopathological factors, high H/I remained statistically significantly associated with patient benefit from letrozole (OR = 0.33; 95% CI = 0.15 to 0.73; P = .006). Reduction in the absolute risk of recurrence at 5 years was 16.5% for patients with high H/I (P = .007). The interaction between H/I and letrozole treatment was statistically significant (P = .03).
In the absence of extended letrozole therapy, high H/I identifies a subgroup of ER-positive patients disease-free after 5 years of tamoxifen who are at risk for late recurrence. When extended endocrine therapy with letrozole is prescribed, high H/I predicts benefit from therapy and a decreased probability of late disease recurrence.
Patients with hormone receptor–positive early breast cancer have a continuous yearly rate of recurrence extending out to 15 years after having received adjuvant endocrine therapy with tamoxifen for 5 years (1). More than half of the recurrences and about two-thirds of breast cancer deaths in this group will occur beyond 5 years from diagnosis (ie, late recurrences and death) (1). The National Cancer Institute of Canada (NCIC) Clinical Trials Group MA.17 trial was a randomized, placebo-controlled trial demonstrating that extended endocrine therapy with letrozole improves disease-free survival (DFS) (2), distant DFS (DDFS), and overall survival (OS) in disease-free postmenopausal patients with hormonal receptor–positive breast cancer after 5 years of tamoxifen (3).
Although extended antihormonal therapy has become standard practice and is endorsed by international clinical practice guidelines (4,5), understanding which patients will actually benefit from longer treatment is paramount to individualized therapy. The status of primary tumor estrogen receptor (ER) and progesterone receptor (PR) expression has been suggested, but not confirmed, as a way to identify patients who have increased benefit from extended letrozole (5). Patients with ER+ and PR+ tumors have been shown to have improved DFS (P = .02) when compared with patients with ER+ and PR− tumors (6). Currently, however, there are no guideline-accepted biomarkers to stratify hormonal receptor–positive patients for prediction of benefit beyond the 5 years of adjuvant endocrine therapy. In addition, standard clinico-pathological factors and clinically available genomic signatures, including the 21-gene (Recurrence Score) assay (7) and the 70-gene assay (8), have greatest prognostic performance for recurrence risk within the first 5 years of adjuvant therapy. Identification of additional biomarkers to further stratify hormone receptor–positive tumors to predict those at risk of late recurrence and those who may or may not benefit from extended endocrine therapy would be of substantial clinical utility (9).
We have previously demonstrated that the two-gene expression ratio, HOXB13/IL17BR (H/I), is a prognostic biomarker in both untreated and tamoxifen-treated early-stage ER+ breast cancer patients (10–13). However, potential assessment of H/I as a predictive biomarker for endocrine therapy has been limited by the analysis of retrospective/observational cohorts or by the use of single treatment arm cohorts from randomized trials (11–13). Herein, we have conducted a prospective–retrospective (14), nested case-control study in a subset of patients from NCIC Clinical Trials Group MA.17 trial to evaluate the performance of H/I for 1) prognostication of late disease recurrence and 2) prediction of treatment benefit from extended adjuvant letrozole therapy in patients with hormone receptor–positive early breast cancer.
Methods
Patients, Case-Control Selection, Study Design, and Endpoints
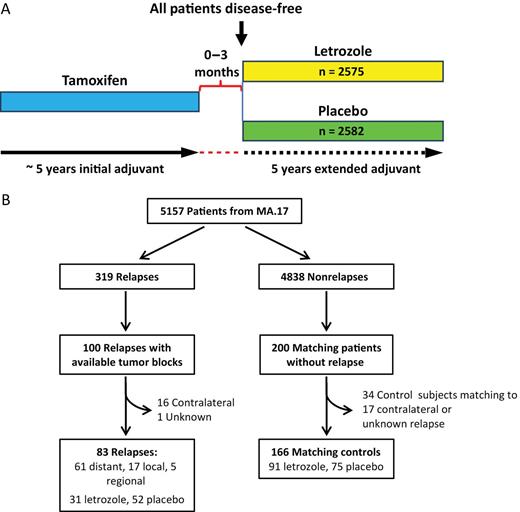
The NCIC Clinical Trials Group MA.17 study was a prospective, randomized, double-blind, placebo-controlled, phase III trial of letrozole as extended adjuvant therapy for 5 years in postmenopausal breast cancer patients who remained disease-free after having completed approximately 5 years of standard adjuvant tamoxifen treatment (Figure 1A). Details of this study have been reported previously (2). Briefly a total of 5157 eligible postmenopausal women with hormone receptor–positive (ER+ and/or PR+) tumors were randomly assigned to receive either letrozole or placebo after completing approximately 5 years of standard adjuvant tamoxifen therapy. In accordance with trial protocol, the MA.17 trial was unblinded in 2003 after the first interim analysis (median follow-up of 30 months) demonstrated a statistically significant disease-free survival benefit and a trend toward survival advantage in patients who received letrozole. Patients on the placebo arm were offered letrozole for a planned period of 5 years.
The overall MA.17 trial design (A), and the sample consort of the MA.17 biomarker study (B).
Because of the practical obstacles to retrospectively obtain primary tumor blocks from MA.17 participants, a nested case-control design using all available recurrent case subjects with a planned ratio of 1:2 was used to study the ability of H/I to predict late recurrence and the benefit from extended therapy with letrozole. Case subjects included patients with local, regional, or distant recurrence for which primary tumor tissue blocks were available. For each case subject, two matched control subjects were selected in which the control subject had been recurrence-free for a period of time longer than the case subject. All patients with disease recurrence and with available formalin-fixed paraffin-embedded (FFPE) tumor tissue blocks were included in this study. Detailed methods for case-control selection are described in Supplementary Methods (available online). The prespecified objectives of this study were to determine whether H/I groups [based on a prespecified and validated cut point (11–13)] would predict late disease recurrence and responsiveness/benefit from extended letrozole therapy after 5 years tamoxifen treatment. The primary endpoint was any breast cancer recurrence, including breast, chest wall, nodal, or metastatic site. Informed consent for the use of tumor specimens for correlative science studies were obtained from each subject. The Massachusetts General Hospital Institutional Review Board approved the use of the MA.17 tumor samples for this study.
Sample Preparation and Pathological Evaluation
Tumor size and grade, lymph node status, and ER and PR status were assessed using local institutional pathology laboratory criteria. Central tumor grade was assessed using the Elston and Ellis modified version of the Bloom and Richardson method by a single subspecialty-trained breast pathologist (D.C. Sgroi). Central review for ER, PR, and HER-2 expression by immunohistochemistry (IHC) and HER gene amplification by fluorescence in situ hybridization was scored by two pathologists (D.C. Sgroi and A.K. Bhan) using two 1.0-mm tissue microarray cores using previously described methods summarized in the Supplementary Material (available online) (15,16).
Reverse-Transcription Polymerase Chain Reaction (RT-PCR) Analysis and Calculation of H/I
Gene expression analysis of formalin-fixed paraffin-embedded specimens was performed blinded to clinical outcome by bioTheranostics Inc, as previously described (11–13). Briefly, hematoxylin and eosin–stained slides were reviewed to guide manual microdissection for tumor enrichment if necessary. After RNA extraction from three to six 8-µm unstained tissue sections and DNase treatment, total RNA content was measured and the absence of DNA contamination verified as previously described (11,13). Total RNA was reverse transcribed, and the resulting cDNA was preamplified by performing eight rounds of PCR using the PreAmp Master Mix Kit (Applied Biosystems, Foster City, CA) before being analyzed by TaqMan RT-PCR. H/I was calculated as previously described (11–13). High and low H/I groups were determined using a prespecified cutpoint (0.06) that was determined and validated in our previous studies (11–13). Reportable results were generated using RNA derived from all 249 formalin-fixed paraffin-embedded tumor samples submitted for testing.
Statistical Analysis
Because of the nature of matched case-control study, unadjusted conditional logistic regression that takes matching set into consideration was used to examine whether H/I was prognostic of late disease recurrence and whether H/I predicts benefit from extended letrozole therapy by including an interaction term between the treatment and predefined H/I groups in the model and testing with statistical contrasts (17). Conditional logistic regression was also performed in an adjusted analysis that also included the following prespecified covariates: age, tumor size, tumor grade, lymph node status, and ER, PR, and HER2 status. Similarly, the predictive analyses in clinically relevant patient subgroups was performed by conditional logistic regressions with a three-way interaction term that included the clinical variable of interest in addition to the treatment and H/I groups. Odds ratios (ORs) and the 95% confidence intervals (CIs) were estimated from conditional logistic regressions.
The absolute recurrence-free survival (RFS) at 5 years and the corresponding 95% CI were estimated using methods developed by Langholz and Borgan (18) for nested case-control study by taking into consideration the number of patients at risk from the entire MA.17 trial at the time of each recurrence in the case-control study. Details on the method are summarized in the Supplementary Methods (available online).
Statistical significance of the interaction between extended letrozole treatment and H/I index as a continuous variable was assessed by a likelihood ratio test that compared a reduced model without H/I index by treatment interaction with the competing full model, which included the interaction. Linearity of H/I index was tested by a likelihood ratio test that compared a model including the H/I index transformed by restricted cubic spline (19) with a model including only the linear H/I index. If a significant nonlinearity existed, then all analyses involving continuous H/I were based on H/I index transformed by restricted cubic spline. All P values were two-sided, and a P value less than .05 was considered statistically significant. All analyses were performed using R statistical package (version 2.12.2, http://www.r-project.org), except for absolute risk estimation for which SAS software (SAS Institute Inc, Cary, NC) was used. All statistical tests were two-sided.
Results
Study Design and Patient and Tumor Characteristics
Tumor samples from 100 patients with disease recurrence were obtained retrospectively and matched to 200 patients without disease recurrence. Because the predefined cutpoint of H/I was previously tested with samples associated with patient outcome defined as local, regional, or distant metastasis (11–13), patients with contralateral and unknown recurrences were removed. Therefore our final cohort consisted of 83 patients with local, regional, or distant recurrence matched with 166 patients without recurrence (Figure 1B). A post hoc power analysis indicated that with this sample size, the power to detect an odds ratio of 3 for comparing risk of late recurrence without extended letrozole vs that with letrozole in the H/I-high group was 82% at a 5% significance level. Characteristics of the case-control population can be seen in Table 1. The majority of patients was aged greater than 50 years with T1/T2 stage disease, and a larger proportion was node-positive and did not receive adjuvant chemotherapy. Within unmatched variables, there was a statistically significant difference between the number of case subjects and control subjects for letrozole vs placebo and high vs low H/I groups (Table 1). Case subjects and control subjects are similar to the overall MA.17 study population with respect to key clinical parameters with the exception of nodal status and radiation therapy (Supplementary Table 1, available online).
Patient clinical and pathological characteristics*
| Characteristics . | Case subjects (n = 83), No. (%) . | Control subjects (n = 166), No. (%) . | P† . |
|---|---|---|---|
| Matched | |||
| Age at diagnosis | |||
| <50 y | 4 (5) | 5 (3) | .63 |
| 50–59 y | 27 (32) | 56 (34) | |
| 60–69 y | 24 (29) | 58 (35) | |
| ≥70 y | 28 (34) | 47 (28) | |
| Tumor stage | |||
| T1 | 37 (45) | 73 (44) | .58 |
| T2 | 35 (42) | 76 (46) | |
| T3 | 7 (8) | 14 (8) | |
| T4, Tx | 4 (5) | 3 (2) | |
| Node status | |||
| Negative | 31 (37) | 63 (38) | .73 |
| Positive | 48 (58) | 98 (59) | |
| Unknown | 4 (5) | 5 (3) | |
| Prior adjuvant chemotherapy | |||
| No | 49 (59) | 99 (60) | 1.00 |
| Yes | 34 (41) | 67 (40) | |
| Unmatched | |||
| Tumor grade | |||
| Well | 6 (7) | 20 (12) | .32 |
| Moderate | 54 (65) | 112 (67.5) | |
| Poor | 23 (27) | 34 (20.5) | |
| ER status | |||
| Positive | 80 (96) | 162 (98) | .69 |
| Negative | 3 (4) | 4 (2) | |
| PR status | |||
| Positive | 68 (82) | 135 (81) | 1.00 |
| Negative | 15 (18) | 31 (19) | |
| HER2 status | |||
| Positive | 9 (11) | 14 (8) | .64 |
| Negative | 74 (89) | 152 (92) | |
| Type of surgery | |||
| Lumpectomy | 51 (61) | 101 (61) | 1.00 |
| Mastectomy | 41 (49) | 88 (53) | .69 |
| Axillary-node dissection | 76 (92) | 161 (97) | .12 |
| Prior adjuvant radiation therapy | |||
| No | 49 (59) | 101 (61) | .79 |
| Yes | 34 (41) | 65 (39) | |
| Treatment | |||
| Letrozole | 31 (37) | 91 (55) | .01 |
| Placebo | 52 (63) | 75 (45) | |
| H/I group‡ | |||
| Low | 35 (42) | 93 (56) | .04 |
| High | 48 (58) | 73 (44) | |
| Characteristics . | Case subjects (n = 83), No. (%) . | Control subjects (n = 166), No. (%) . | P† . |
|---|---|---|---|
| Matched | |||
| Age at diagnosis | |||
| <50 y | 4 (5) | 5 (3) | .63 |
| 50–59 y | 27 (32) | 56 (34) | |
| 60–69 y | 24 (29) | 58 (35) | |
| ≥70 y | 28 (34) | 47 (28) | |
| Tumor stage | |||
| T1 | 37 (45) | 73 (44) | .58 |
| T2 | 35 (42) | 76 (46) | |
| T3 | 7 (8) | 14 (8) | |
| T4, Tx | 4 (5) | 3 (2) | |
| Node status | |||
| Negative | 31 (37) | 63 (38) | .73 |
| Positive | 48 (58) | 98 (59) | |
| Unknown | 4 (5) | 5 (3) | |
| Prior adjuvant chemotherapy | |||
| No | 49 (59) | 99 (60) | 1.00 |
| Yes | 34 (41) | 67 (40) | |
| Unmatched | |||
| Tumor grade | |||
| Well | 6 (7) | 20 (12) | .32 |
| Moderate | 54 (65) | 112 (67.5) | |
| Poor | 23 (27) | 34 (20.5) | |
| ER status | |||
| Positive | 80 (96) | 162 (98) | .69 |
| Negative | 3 (4) | 4 (2) | |
| PR status | |||
| Positive | 68 (82) | 135 (81) | 1.00 |
| Negative | 15 (18) | 31 (19) | |
| HER2 status | |||
| Positive | 9 (11) | 14 (8) | .64 |
| Negative | 74 (89) | 152 (92) | |
| Type of surgery | |||
| Lumpectomy | 51 (61) | 101 (61) | 1.00 |
| Mastectomy | 41 (49) | 88 (53) | .69 |
| Axillary-node dissection | 76 (92) | 161 (97) | .12 |
| Prior adjuvant radiation therapy | |||
| No | 49 (59) | 101 (61) | .79 |
| Yes | 34 (41) | 65 (39) | |
| Treatment | |||
| Letrozole | 31 (37) | 91 (55) | .01 |
| Placebo | 52 (63) | 75 (45) | |
| H/I group‡ | |||
| Low | 35 (42) | 93 (56) | .04 |
| High | 48 (58) | 73 (44) | |
* All statistical tests were two-sided. ER = estrogen receptor; H/I = HOXB13/IL17BR; PR = progesterone receptor.
† P values were calculated using the Fisher exact test, except for “Type of surgery,” for which binomial proportional test was used.
‡ High and low H/I groups were determined using a prespecified cutpoint (0.06) that was determined and validated in our previous studies (11–13).
Patient clinical and pathological characteristics*
| Characteristics . | Case subjects (n = 83), No. (%) . | Control subjects (n = 166), No. (%) . | P† . |
|---|---|---|---|
| Matched | |||
| Age at diagnosis | |||
| <50 y | 4 (5) | 5 (3) | .63 |
| 50–59 y | 27 (32) | 56 (34) | |
| 60–69 y | 24 (29) | 58 (35) | |
| ≥70 y | 28 (34) | 47 (28) | |
| Tumor stage | |||
| T1 | 37 (45) | 73 (44) | .58 |
| T2 | 35 (42) | 76 (46) | |
| T3 | 7 (8) | 14 (8) | |
| T4, Tx | 4 (5) | 3 (2) | |
| Node status | |||
| Negative | 31 (37) | 63 (38) | .73 |
| Positive | 48 (58) | 98 (59) | |
| Unknown | 4 (5) | 5 (3) | |
| Prior adjuvant chemotherapy | |||
| No | 49 (59) | 99 (60) | 1.00 |
| Yes | 34 (41) | 67 (40) | |
| Unmatched | |||
| Tumor grade | |||
| Well | 6 (7) | 20 (12) | .32 |
| Moderate | 54 (65) | 112 (67.5) | |
| Poor | 23 (27) | 34 (20.5) | |
| ER status | |||
| Positive | 80 (96) | 162 (98) | .69 |
| Negative | 3 (4) | 4 (2) | |
| PR status | |||
| Positive | 68 (82) | 135 (81) | 1.00 |
| Negative | 15 (18) | 31 (19) | |
| HER2 status | |||
| Positive | 9 (11) | 14 (8) | .64 |
| Negative | 74 (89) | 152 (92) | |
| Type of surgery | |||
| Lumpectomy | 51 (61) | 101 (61) | 1.00 |
| Mastectomy | 41 (49) | 88 (53) | .69 |
| Axillary-node dissection | 76 (92) | 161 (97) | .12 |
| Prior adjuvant radiation therapy | |||
| No | 49 (59) | 101 (61) | .79 |
| Yes | 34 (41) | 65 (39) | |
| Treatment | |||
| Letrozole | 31 (37) | 91 (55) | .01 |
| Placebo | 52 (63) | 75 (45) | |
| H/I group‡ | |||
| Low | 35 (42) | 93 (56) | .04 |
| High | 48 (58) | 73 (44) | |
| Characteristics . | Case subjects (n = 83), No. (%) . | Control subjects (n = 166), No. (%) . | P† . |
|---|---|---|---|
| Matched | |||
| Age at diagnosis | |||
| <50 y | 4 (5) | 5 (3) | .63 |
| 50–59 y | 27 (32) | 56 (34) | |
| 60–69 y | 24 (29) | 58 (35) | |
| ≥70 y | 28 (34) | 47 (28) | |
| Tumor stage | |||
| T1 | 37 (45) | 73 (44) | .58 |
| T2 | 35 (42) | 76 (46) | |
| T3 | 7 (8) | 14 (8) | |
| T4, Tx | 4 (5) | 3 (2) | |
| Node status | |||
| Negative | 31 (37) | 63 (38) | .73 |
| Positive | 48 (58) | 98 (59) | |
| Unknown | 4 (5) | 5 (3) | |
| Prior adjuvant chemotherapy | |||
| No | 49 (59) | 99 (60) | 1.00 |
| Yes | 34 (41) | 67 (40) | |
| Unmatched | |||
| Tumor grade | |||
| Well | 6 (7) | 20 (12) | .32 |
| Moderate | 54 (65) | 112 (67.5) | |
| Poor | 23 (27) | 34 (20.5) | |
| ER status | |||
| Positive | 80 (96) | 162 (98) | .69 |
| Negative | 3 (4) | 4 (2) | |
| PR status | |||
| Positive | 68 (82) | 135 (81) | 1.00 |
| Negative | 15 (18) | 31 (19) | |
| HER2 status | |||
| Positive | 9 (11) | 14 (8) | .64 |
| Negative | 74 (89) | 152 (92) | |
| Type of surgery | |||
| Lumpectomy | 51 (61) | 101 (61) | 1.00 |
| Mastectomy | 41 (49) | 88 (53) | .69 |
| Axillary-node dissection | 76 (92) | 161 (97) | .12 |
| Prior adjuvant radiation therapy | |||
| No | 49 (59) | 101 (61) | .79 |
| Yes | 34 (41) | 65 (39) | |
| Treatment | |||
| Letrozole | 31 (37) | 91 (55) | .01 |
| Placebo | 52 (63) | 75 (45) | |
| H/I group‡ | |||
| Low | 35 (42) | 93 (56) | .04 |
| High | 48 (58) | 73 (44) | |
* All statistical tests were two-sided. ER = estrogen receptor; H/I = HOXB13/IL17BR; PR = progesterone receptor.
† P values were calculated using the Fisher exact test, except for “Type of surgery,” for which binomial proportional test was used.
‡ High and low H/I groups were determined using a prespecified cutpoint (0.06) that was determined and validated in our previous studies (11–13).
The observed clinical benefit from letrozole within different patient subgroups was similar between the case-control study and the entire study population reported in the MA.17 trial (Table 2). The benefit was statistically significant in both studies in all subgroups except within the lymph node–positive subgroup for the case-control, which may be because of small sample size for the relatively smaller size of the benefit (vs lymph node negative) (Table 2). For example, the odds ratio estimated by conditional logistic regression with an interaction between clinical factors and the treatment in this case-control study and the hazard ratio estimated from the overall MA.17 trial as in published literature (6,20) were as follows: 0.20 and 0.45 for node negative, 0.63 and 0.61 for node positive, 0.44 and 0.73 for aged greater or equal to 50 years, 0.42 and 0.58 for hormone receptor positive, and 0.45 and 0.49 for ER+/PR+ (Table 2).
Comparison of clinical benefit from extended letrozole therapy in patient subgroups of the nested case-control study and the overall MA.17 trial*
| Patient subgroups . | Nested case-control study (n = 249) . | Overall MA.17 trial (n = 5157) . |
|---|---|---|
| OR (95% CI) . | HR (95% CI) . | |
| Node-negative | 0.20 (0.07 to 0.63) | 0.45 (0.27 to 0.73) |
| Node-positive | 0.63 (0.31 to 1.28) | 0.61 (0.45 to 0.84) |
| Aged ≥ 50 y | 0.44 (0.24 to 0.78) | 0.73 (0.55 to 0.98) |
| ER-positive or PR-positive | 0.42 (0.23 to 0.74) | 0.58 (0.45 to 0.76) |
| ER-positive and PR-positive | 0.45 (0.24 to 0.84) | 0.49 (0.36 to 0.67) |
| Patient subgroups . | Nested case-control study (n = 249) . | Overall MA.17 trial (n = 5157) . |
|---|---|---|
| OR (95% CI) . | HR (95% CI) . | |
| Node-negative | 0.20 (0.07 to 0.63) | 0.45 (0.27 to 0.73) |
| Node-positive | 0.63 (0.31 to 1.28) | 0.61 (0.45 to 0.84) |
| Aged ≥ 50 y | 0.44 (0.24 to 0.78) | 0.73 (0.55 to 0.98) |
| ER-positive or PR-positive | 0.42 (0.23 to 0.74) | 0.58 (0.45 to 0.76) |
| ER-positive and PR-positive | 0.45 (0.24 to 0.84) | 0.49 (0.36 to 0.67) |
* Odds ratio (OR) and 95% confidence interval (CI) were calculated from conditional logistic regression. All statistical tests were two-sided. ER = estrogen receptor; HR = hazard ratio; PR = progesterone receptor.
Comparison of clinical benefit from extended letrozole therapy in patient subgroups of the nested case-control study and the overall MA.17 trial*
| Patient subgroups . | Nested case-control study (n = 249) . | Overall MA.17 trial (n = 5157) . |
|---|---|---|
| OR (95% CI) . | HR (95% CI) . | |
| Node-negative | 0.20 (0.07 to 0.63) | 0.45 (0.27 to 0.73) |
| Node-positive | 0.63 (0.31 to 1.28) | 0.61 (0.45 to 0.84) |
| Aged ≥ 50 y | 0.44 (0.24 to 0.78) | 0.73 (0.55 to 0.98) |
| ER-positive or PR-positive | 0.42 (0.23 to 0.74) | 0.58 (0.45 to 0.76) |
| ER-positive and PR-positive | 0.45 (0.24 to 0.84) | 0.49 (0.36 to 0.67) |
| Patient subgroups . | Nested case-control study (n = 249) . | Overall MA.17 trial (n = 5157) . |
|---|---|---|
| OR (95% CI) . | HR (95% CI) . | |
| Node-negative | 0.20 (0.07 to 0.63) | 0.45 (0.27 to 0.73) |
| Node-positive | 0.63 (0.31 to 1.28) | 0.61 (0.45 to 0.84) |
| Aged ≥ 50 y | 0.44 (0.24 to 0.78) | 0.73 (0.55 to 0.98) |
| ER-positive or PR-positive | 0.42 (0.23 to 0.74) | 0.58 (0.45 to 0.76) |
| ER-positive and PR-positive | 0.45 (0.24 to 0.84) | 0.49 (0.36 to 0.67) |
* Odds ratio (OR) and 95% confidence interval (CI) were calculated from conditional logistic regression. All statistical tests were two-sided. ER = estrogen receptor; HR = hazard ratio; PR = progesterone receptor.
Prognostication of Late Disease Recurrence by H/I
In the placebo arm, H/I status was statistically significantly associated with prognosis in the unadjusted model, as women in the high H/I group experienced substantially worse prognosis than those in the low H/I group (OR = 2.24; 95% CI = 1.09 to 4.61; P = .03). H/I status remained marginally statistically significant in the adjusted model (OR = 2.15; 95% CI = 1.00 to 4.64; P = .05) after adjusting for all clinicopathological factors examined (age, tumor size, tumor grade, nodal status, ER, PR, HER2) (Supplementary Table 2). In the letrozole arm, however, H/I was not prognostic for late recurrence in neither the unadjusted nor the adjusted models (P = .72 and .63, respectively).
Predictive Performance of H/I for Benefit From Letrozole Treatment
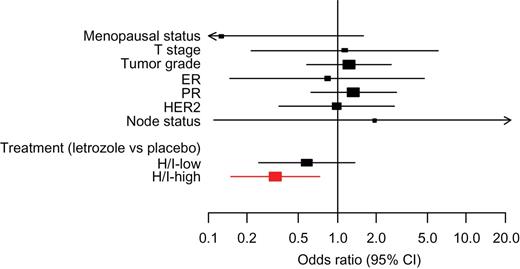
In the unadjusted analysis, high H/I group was statistically significantly associated with patient benefit from letrozole (OR = 0.35; 95% CI = 0.16 to 0.75; P = .007) (Table 3). In the adjusted model, which included all clinicopathological factors as covariates, high H/I remained statistically significantly associated with patient benefit from letrozole (OR = 0.33; 95% CI = 0.15 to 0.73; P = .006) (Table 3; Figure 2), which represented a 67% reduction in the risk of recurrence with extended letrozole treatment as compared with placebo.
Unadjusted and adjusted analyses of treatment benefit by HOXB13/IL17BR (H/I) groups
| Variable . | Unadjusted OR (95% CI) . | P . | Adjusted OR (95% CI) . | P . |
|---|---|---|---|---|
| Age, post- vs premenopausal | 0.25 (0.02 to 2.76) | .26 | 0.13 (0.01 to 1.60) | .11 |
| Tumor size, T2 + T3 vs T1 | 1.00 (0.23 to 4.35) | 1.00 | 1.13 (0.21 to 6.00) | .88 |
| Grade, 3 vs 1–2 | 1.56 (0.82 to 2.98) | .18 | 1.23 (0.58 to 2.60) | .59 |
| ER status, positive vs negative | 0.67 (0.15 to 2.98) | .60 | 0.83 (0.15 to 4.72) | .83 |
| PR status, positive vs negative | 1.05 (0.53 to 2.09) | .88 | 1.33 (0.62 to 2.86) | .46 |
| HER2 status, positive vs negative | 1.32 (0.55 to 3.18) | .54 | 0.99 (0.35 to 2.78) | .98 |
| Node status, positive vs negative | 1.00 (0.06 to 15.99) | 1.00 | 1.93 (0.11 to 33.77) | .65 |
| Treatment effect, letrozole vs placebo | ||||
| H/I-low | 0.68 (0.31 to 1.52) | .35 | 0.58 (0.25 to 1.36) | .21 |
| H/I-high | 0.35 (0.16 to 0.75) | .007 | 0.33 (0.15 to 0.73) | .006 |
| Variable . | Unadjusted OR (95% CI) . | P . | Adjusted OR (95% CI) . | P . |
|---|---|---|---|---|
| Age, post- vs premenopausal | 0.25 (0.02 to 2.76) | .26 | 0.13 (0.01 to 1.60) | .11 |
| Tumor size, T2 + T3 vs T1 | 1.00 (0.23 to 4.35) | 1.00 | 1.13 (0.21 to 6.00) | .88 |
| Grade, 3 vs 1–2 | 1.56 (0.82 to 2.98) | .18 | 1.23 (0.58 to 2.60) | .59 |
| ER status, positive vs negative | 0.67 (0.15 to 2.98) | .60 | 0.83 (0.15 to 4.72) | .83 |
| PR status, positive vs negative | 1.05 (0.53 to 2.09) | .88 | 1.33 (0.62 to 2.86) | .46 |
| HER2 status, positive vs negative | 1.32 (0.55 to 3.18) | .54 | 0.99 (0.35 to 2.78) | .98 |
| Node status, positive vs negative | 1.00 (0.06 to 15.99) | 1.00 | 1.93 (0.11 to 33.77) | .65 |
| Treatment effect, letrozole vs placebo | ||||
| H/I-low | 0.68 (0.31 to 1.52) | .35 | 0.58 (0.25 to 1.36) | .21 |
| H/I-high | 0.35 (0.16 to 0.75) | .007 | 0.33 (0.15 to 0.73) | .006 |
* Odds ratio (OR), 95% confidence interval (CI), and P values were calculated from conditional logistic regression. All statistical tests were two-sided. ER = estrogen receptor; PR = progesterone receptor.
Unadjusted and adjusted analyses of treatment benefit by HOXB13/IL17BR (H/I) groups
| Variable . | Unadjusted OR (95% CI) . | P . | Adjusted OR (95% CI) . | P . |
|---|---|---|---|---|
| Age, post- vs premenopausal | 0.25 (0.02 to 2.76) | .26 | 0.13 (0.01 to 1.60) | .11 |
| Tumor size, T2 + T3 vs T1 | 1.00 (0.23 to 4.35) | 1.00 | 1.13 (0.21 to 6.00) | .88 |
| Grade, 3 vs 1–2 | 1.56 (0.82 to 2.98) | .18 | 1.23 (0.58 to 2.60) | .59 |
| ER status, positive vs negative | 0.67 (0.15 to 2.98) | .60 | 0.83 (0.15 to 4.72) | .83 |
| PR status, positive vs negative | 1.05 (0.53 to 2.09) | .88 | 1.33 (0.62 to 2.86) | .46 |
| HER2 status, positive vs negative | 1.32 (0.55 to 3.18) | .54 | 0.99 (0.35 to 2.78) | .98 |
| Node status, positive vs negative | 1.00 (0.06 to 15.99) | 1.00 | 1.93 (0.11 to 33.77) | .65 |
| Treatment effect, letrozole vs placebo | ||||
| H/I-low | 0.68 (0.31 to 1.52) | .35 | 0.58 (0.25 to 1.36) | .21 |
| H/I-high | 0.35 (0.16 to 0.75) | .007 | 0.33 (0.15 to 0.73) | .006 |
| Variable . | Unadjusted OR (95% CI) . | P . | Adjusted OR (95% CI) . | P . |
|---|---|---|---|---|
| Age, post- vs premenopausal | 0.25 (0.02 to 2.76) | .26 | 0.13 (0.01 to 1.60) | .11 |
| Tumor size, T2 + T3 vs T1 | 1.00 (0.23 to 4.35) | 1.00 | 1.13 (0.21 to 6.00) | .88 |
| Grade, 3 vs 1–2 | 1.56 (0.82 to 2.98) | .18 | 1.23 (0.58 to 2.60) | .59 |
| ER status, positive vs negative | 0.67 (0.15 to 2.98) | .60 | 0.83 (0.15 to 4.72) | .83 |
| PR status, positive vs negative | 1.05 (0.53 to 2.09) | .88 | 1.33 (0.62 to 2.86) | .46 |
| HER2 status, positive vs negative | 1.32 (0.55 to 3.18) | .54 | 0.99 (0.35 to 2.78) | .98 |
| Node status, positive vs negative | 1.00 (0.06 to 15.99) | 1.00 | 1.93 (0.11 to 33.77) | .65 |
| Treatment effect, letrozole vs placebo | ||||
| H/I-low | 0.68 (0.31 to 1.52) | .35 | 0.58 (0.25 to 1.36) | .21 |
| H/I-high | 0.35 (0.16 to 0.75) | .007 | 0.33 (0.15 to 0.73) | .006 |
* Odds ratio (OR), 95% confidence interval (CI), and P values were calculated from conditional logistic regression. All statistical tests were two-sided. ER = estrogen receptor; PR = progesterone receptor.
Forest plots showing odds ratio (OR) for recurrence associated with clinico-pathological factors and treatment effect for each of the HOXB13/IL17BR (H/I) groups. The odds ratio for recurrence was calculated from conditional logistic regression. Red color indicates H/I-high patients; black color indicates H/I-low patients. All statistical tests were two-sided. CI = confidence interval; ER = estrogen receptor; PR = progesterone receptor.
The absolute RFS at 4 years estimated from this case-control study was 86.6% (95% CI = 80.6% to 90.9%) and 93.4% (95% CI = 90.2% to 95.6%) for patients receiving placebo and letrozole, respectively. This is consistent with the 4-year disease-free survival (87% and 93% for placebo and letrozole, respectively) estimated from the parental MA.17 trial (2). For low H/I patients, the 5-year RFS was 87% (95% CI = 76.8% to 92.9%) and 91% (95% CI = 83.1% to 95.3%) in the placebo and letrozole group, respectively, showing a non-statistically significant reduction in the risk of recurrence of 4% (P = .35). However, for high H/I patients, the 5-year RFS was 73% (95% CI = 56.6% to 84.1%) and 89.5% (95% CI = 80.3% to 94.5%) in the placebo and letrozole group, demonstrating a 16.5% reduction in the absolute risk of recurrence at 5 years (P = .007) (Table 4).
Estimates of recurrence-free survival (RFS) at 5 years in patients who were treated with placebo or extended letrozole
| Patient subgroups . | Placebo . | Letrozole . | ||
|---|---|---|---|---|
| No. of patients (%) . | 5-Year RFS (95% CI, %) . | No. of patients (%) . | 5-Year RFS (95% CI, %) . | |
| All patients | 127 (100) | 80.4 (68.0 to 88.4) | 122 (100) | 90.1 (82.3 to 94.6) |
| H/I-low | 65 (51) | 87.0 (76.8 to 92.9) | 63 (52) | 91.0 (83.1 to 95.3) |
| H/I-high | 62 (49) | 73.0 (56.6 to 84.1) | 59 (48) | 89.5 (80.3 to 94.5) |
| Patient subgroups . | Placebo . | Letrozole . | ||
|---|---|---|---|---|
| No. of patients (%) . | 5-Year RFS (95% CI, %) . | No. of patients (%) . | 5-Year RFS (95% CI, %) . | |
| All patients | 127 (100) | 80.4 (68.0 to 88.4) | 122 (100) | 90.1 (82.3 to 94.6) |
| H/I-low | 65 (51) | 87.0 (76.8 to 92.9) | 63 (52) | 91.0 (83.1 to 95.3) |
| H/I-high | 62 (49) | 73.0 (56.6 to 84.1) | 59 (48) | 89.5 (80.3 to 94.5) |
* RFSs and 95% confidence intervals (CIs) were calculated from conditional logistic regression using the method developed by Langholz and Borgan for nested case-control study. All statistical tests were two-sided. H/I = HOXB13/IL17BR.
Estimates of recurrence-free survival (RFS) at 5 years in patients who were treated with placebo or extended letrozole
| Patient subgroups . | Placebo . | Letrozole . | ||
|---|---|---|---|---|
| No. of patients (%) . | 5-Year RFS (95% CI, %) . | No. of patients (%) . | 5-Year RFS (95% CI, %) . | |
| All patients | 127 (100) | 80.4 (68.0 to 88.4) | 122 (100) | 90.1 (82.3 to 94.6) |
| H/I-low | 65 (51) | 87.0 (76.8 to 92.9) | 63 (52) | 91.0 (83.1 to 95.3) |
| H/I-high | 62 (49) | 73.0 (56.6 to 84.1) | 59 (48) | 89.5 (80.3 to 94.5) |
| Patient subgroups . | Placebo . | Letrozole . | ||
|---|---|---|---|---|
| No. of patients (%) . | 5-Year RFS (95% CI, %) . | No. of patients (%) . | 5-Year RFS (95% CI, %) . | |
| All patients | 127 (100) | 80.4 (68.0 to 88.4) | 122 (100) | 90.1 (82.3 to 94.6) |
| H/I-low | 65 (51) | 87.0 (76.8 to 92.9) | 63 (52) | 91.0 (83.1 to 95.3) |
| H/I-high | 62 (49) | 73.0 (56.6 to 84.1) | 59 (48) | 89.5 (80.3 to 94.5) |
* RFSs and 95% confidence intervals (CIs) were calculated from conditional logistic regression using the method developed by Langholz and Borgan for nested case-control study. All statistical tests were two-sided. H/I = HOXB13/IL17BR.
To examine whether continuous ER and PR measurements would affect the predictive performance of H/I, gene expression values of ER and PR by RT-PCR were used in the adjusted model instead of binary immunohistochemistry ER and PR values. High H/I remained statistically significant with extended letrozole benefit (OR = 0.32; 95% CI = 0.14 to 0.72; P = .006), whereas low H/I still showed no statistically significant benefit (OR = 0.59; 95% CI = 0.25 to 1.39; P = .23).
Linearity testing with a conditional logistic regression model indicated that there was a significant nonlinear relationship of H/I index as a continuous variable with recurrence (P = .02); therefore H/I index was transformed by restricted cubic spline when assessing the statistical significance of the interaction between the treatment and continuous H/I index. Likelihood ratio test showed that the interaction between H/I and letrozole therapy was statistically significant (P = 0.03), adjusting for clinicopathological factors.
Predictive Performance of H/I Within Clinically Relevant Subgroups
Unplanned analyses were conducted to determine whether patient subgroups were differentially associated with the predictive performance of H/I by including a conditional logistic regression three-way interaction term between the clinical variable of interest, treatment, and H/I groups. High H/I was statistically significantly predictive of letrozole benefit in our overall study population. Analyses of subsets including nodal, HER2, ER, and PR status are shown in Supplementary Table 3 and Supplementary Figure 1 (available online).
Discussion
Currently, for ER-positive breast cancer patients, there are no established tests that predict treatment benefit from extended adjuvant endocrine therapy (15). In this prospectively defined, retrospective analysis of a nested case-controlled study of MA.17, patients with high H/I–expressing tumors had a 67% reduction in relative risk of recurrence and 16.5% reduction in the absolute risk of recurrence at 5 years when taking letrozole, as compared with placebo. Notably, the strong consistency in the estimates of 4-year RFS in both treatment groups calculated from this case-control study vs those from the parental MA.17 study population contends that the case-control samples are representative of the entire MA.17 study population. In addition, there was a statistically significant interaction between H/I as a continuous index and extended aromatase inhibitor (AI) treatment. As a comparison, continuous ER and PR values measured by RT-PCR had no statistically significant interaction with extended AI treatment (P = .11 and .24, respectively). The prognostic performance of high H/I for late recurrence within the placebo group was offset by the benefit from letrozole therapy for patients with tumors expressing high H/I. These data suggest that high H/I within the primary tumor stratified a group of patients that had a greater likelihood for benefit from extended adjuvant letrozole above the known residual benefit from 5 years of adjuvant tamoxifen therapy. In addition, patients with high H/I in their primary tumor have a greater likelihood of experiencing a recurrence beyond 5 years of tamoxifen treatment if their adjuvant therapy is not extended with letrozole. Although mechanistically unclear, H/I has both prognostic and predictive performance similar to HER2 in that higher expression of the biomarker suggests a worse prognosis for breast cancer patients yet indicates a favorable response to a given therapy.
Our study has limitations. As our retrospective case subject selection was based on available tissue blocks, the size of our case-control cohort is substantially smaller than the overall MA.17 trial, and this selection could have introduced bias. Despite this, apart from fewer patients treated with adjuvant radiation therapy and a greater proportion with lymph node–positive disease, our cohort was similar to the overall MA.17 study population with respect to key clinical parameters (Supplementary Table 1, available online). Moreover, we showed similar benefit from letrozole treatment in our overall case-control cohort and within important subgroups in our study as compared with the overall MA.17 patient population.
The clinical applications of our findings apply to many women with ER+ early breast cancer. First, 5 years of adjuvant tamoxifen remains the standard adjuvant therapy for premenopausal women most of whom are menopausal after chemotherapy and 5 years of tamoxifen and are therefore potential candidates for extended AI therapy. Second, although upfront AI adjuvant therapy has become more common, 5 years of tamoxifen remains a common adjuvant therapy among postmenopausal women globally. Identifying which patients are vulnerable to a late recurrence and who among them will benefit from extended therapy is of substantial clinical value. Our biomarker should allow many women to avoid unnecessary treatment and for the focus to center on those in most need of therapy. This, in turn, could improve compliance with medication and further improve outcomes (21–24).
In conclusion, we have demonstrated in a prospective–retrospective analysis of the MA.17 trial that patients with primary tumors with a high H/I index who are disease-free after 4.5 to 6 years of adjuvant tamoxifen therapy receive benefit from extended adjuvant letrozole therapy. Performance of H/I in predicting treatment benefit from extended AI therapy for women who have used tamoxifen for less than 5 years and those who have taken upfront AI without any tamoxifen for 5 years awaits prospective–retrospective analyses of currently ongoing trials such as MA.17R, NSABP42, and others (25,26).
Funding
This work was supported by grants from the Avon Foundation (to D.C.S. and P.E.G.); the National Institute of Health (R01CA112021 to D.C.S.); the Breast Cancer Foundation (to D.C.S. and P.E.G.); the Department of Defense Breast Cancer Research Program (W81XWH-04-1-0606 to D.C.S.); the NCI SPORE in breast cancer at Massachusetts General Hospital (to P.E.G. and D.C.S.); and Novartis.
D.C. Sgroi was the principal investigator. D.C. Sgroi and P.E. Goss participated in all phases of this study, including design and writing of the biomarker proposal, submission for the Breast Cancer Intergroup of North America Correlative Science Committee/NCI approval, data analysis and interpretation, and preparation of the manuscript. D.M. Finkelstein, J.N. Ingle, P. Porter, H.B. Muss, K.I. Pritchard, and D. Tu participated in study design. E. Carney, E. Zarrella, L. Steffel, and S.N. Binns participated in data collection. D.M. Finkelstein, J. Szymonifka, and Y. Zhang performed the statistical analysis. A.K. Bhan and D.L. Rimm participated in data collection, data analysis, and data interpretation. D.M. Finkelstein, J. Szymonifka, Y. Zhang, C.A. Schnabel, and M.G. Erlander participated in the data analysis and interpretation and preparation of the manuscript. All authors approved the contents of the manuscript.
D.C. Sgroi and M.G. Erlander are named inventors on a patent to use the HOXB13/IL17BR assay to predict breast cancer outcome. P.E. Goss has received occasional honoraria from Novartis and Glaxo-Smith-Kline. K.I. P has received occasional advisory and expert testimony honoraria from Novartis, Roche, Pfizer, Astrazeneca, and Glaxo-Smith-Kline. Y. Zhang, C.A. Schnabel, and M.G. Erlander are full-time employees and stockholders of bioTheranostics. All other authors declare no relevant conflicts of interest.
We thank Drs Pedro Liedke and Soonmyung Paik for helpful discussions and comments, Dr Bryan Langholz for statistical analysis on the estimation of absolute risk of recurrence, and Ranelle Salunga for technical expertise. We are indebted to the women who participated in the MA.17 trial.
References