-
PDF
- Split View
-
Views
-
Cite
Cite
Rizwan Attia, Prem Venugopal, Donald Whitaker, Christopher Young, Management of a pulsatile mass coming through the sternum. Pseudoaneurysm of ascending aorta 35 years after repair of tetralogy of Fallot, Interactive CardioVascular and Thoracic Surgery, Volume 10, Issue 5, May 2010, Pages 820–822, https://doi.org/10.1510/icvts.2009.227900
Close - Share Icon Share
Abstract
We describe a case of an ascending aortic pseudoaneurysm during long-term follow-up after repair of tetralogy of Fallot (TOF). The patient had a complex cardiac surgical history with multiple operations for the correction of TOF. The aneurysm was located at the presumed site of previous aortic cannulation. It was initially treated percutaneously with an Amplatzer™ septal occluder device, with limited early success. After 12 months it was found to have migrated into the sac and open surgical repair was undertaken successfully.
1. Case
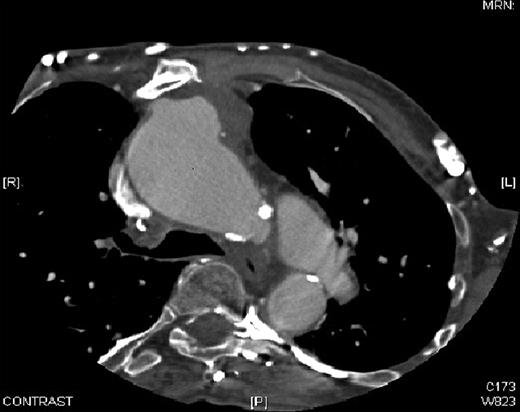
A 64-year-old patient presented with dyspnoea, paroxysmal nocturnal dyspnoea, dysphagia and back pain. She had a complex cardiac surgical history starting with an unsuccessful Pott's shunt for tetralogy of Fallot aged 4. This was followed by a modified Blalock-Taussig (BT) shunt aged 14. A total correction of Fallot (which included a trans annular patch and ligation of right BT shunt) was performed aged 28. Four years later she presented with a pulsating mass in the precordium. The right ventricular outflow tract was aneurysmal. This was excised and repaired. Aged 62 she now presented with a pulsatile mass visible through the sternum. She was diagnosed as having a pseudoaneurysm of the ascending aorta. This was closed with an 18 mm Amplatzer™ muscular ventricular septal defect (VSD) device by interventional cardiology as the risk of open surgical procedure was considered high. The procedure resulted in a satisfactory seal at the neck of the aneurysm. One year later, however, the device was found to have migrated into the pseudoaneurysm (Fig. 1 ).
Computer tomogram demonstrating the Amplatzer™ muscular VSD device displacement into the pseudoaneurysm. The two disks of the circular device are seen eroded through the pseudoaneurysm into the posterior table of sternum. VSD, ventricular septal defect.
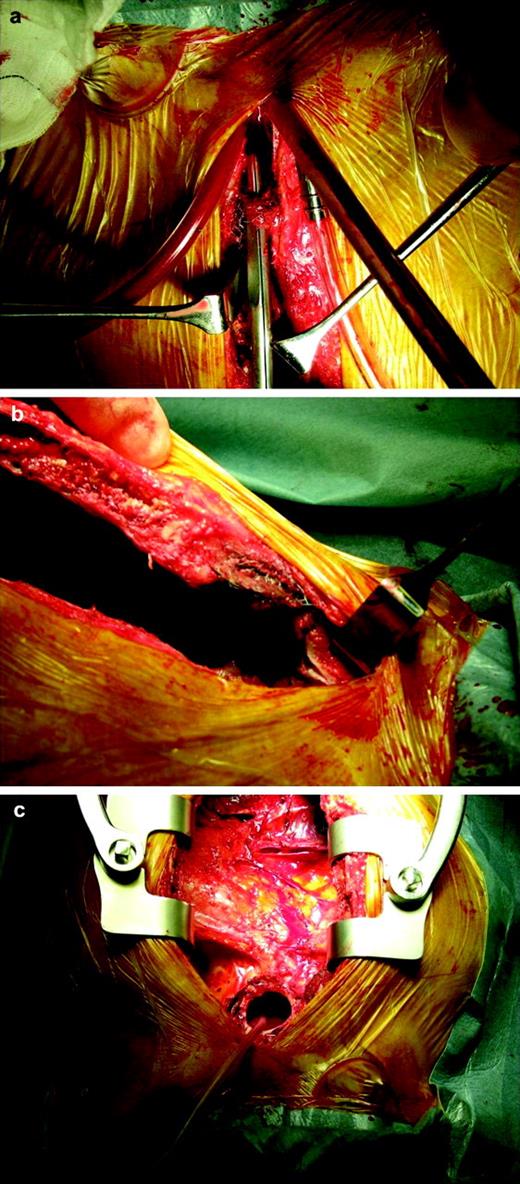
Open repair of the ascending aortic pseudoaneurysm was performed under hypothermic circulatory arrest (HCA). Femoro-femoral cardiopulmonary bypass (CPB) was established. A left sub-mammary incision was made to allow venting of the left ventricular apex. HCA was achieved at 15 degrees. A median sternotomy was performed. The pseudoaneurysm contained thrombus. The Amplatzer™ device was embedded into the wall of the pseudoaneurysm which had eroded into the sternal medullary cavity (Fig. 2 ). Dense adhesions were present throughout the mediastinum. The neck of the aneurysm was 2.5 cm; it was closed with a Haemashield Patch®. Carbondioxide was insufflated throughout circulatory arrest. CPB was re-established and standard de-airing manoeuvres carried out. A Gore-tex® shield was placed over ascending aorta prior to closure, behind the sternum, in case further surgery is required in the future. The heart was weaned off CPB uneventfully without inotropic support (CPB time 156 min and HCA time of 9 min). The postoperative recovery was complicated by a right middle cerebral artery stroke on day 3, from which she recovered well. She remains well at 12 months follow-up.
The device has eroded into the posterior table of sternum. Defect in the ascending aorta visible that required patch closure and exclusion of the aneurysm sac.
2. Discussion
The medical and surgical experience with modified BT shunt and one-stage repair of tetralogy are extensive. However, complications still occur. Pseudoaneurysm development is rare but has been described in the literature in this setting [1]. As far as we are aware this is the first documented case of pseudoaneurysm presenting 35 years after initial intervention. Pseudoaneurysms usually form at the site of aortic cannulation, aortotomy or anastomoses. They can be lethal as they are prone to rupture, thrombosis, distal embolisation and fistula formation. Percutaneous approach to closure of large ascending pseudoaneurysms has been described using Amplatzer™ device [2, 3]. Surgical repair is recommended but may be associated with high morbidity and mortality [4, 5].
Utilising the Amplatzer™ septal occluder has its own challenges. These include the diameter of ascending aorta and angle of approach that make device misalignment likely. The device was oversized to compensate for this. Although initially the device achieved an adequate seal, migration to other sites remains a serious hazard [6]. Continued expansion of the neck of the pseudoaneurysm ultimately led to embolisation of the device into the sac. The Amplatzer™ device provided a temporising measure when the patient initially presented, allowing a more planned operative approach later.
The operative management of ascending aortic pseudoaneurysms remains technically challenging. Mortality rates from 29 to 46% are quoted in the literature. This is usually due to fatal haemorrhage resulting from rupture of the pseudoaneurysm during sternal re-entry or surgical repair techniques [4]. As in our case, median sternotomy with femoro-femoral bypass and HCA has been the strategy of choice [5]. The main tenants of operative approach being prevention against cardiac injury and ensuing exsanguination; decompression of left ventricle during cooling and neurological protection.
Our strategy for chest re-entry was to decompress the left ventricle with an apical vent. Divide the sternum at low flow with the patient fully cooled to 15 degrees and to fully mobilise the right ventricle before reaching the pseudoaneurysm.
There are alternative strategies for reoperation for giant false aneurysm of the thoracic aorta. Bachet et al. [7] describe an alternate useful technique of separate carotid cannulation and selective antegrade cerebral perfusion with cold blood during circulatory arrest at moderate core hypothermia. This technique allows for safe chest re-entry without general deep hypothermia, reduced CPB and HCA times and neurological protection. The mean CPB and HCA time in their series was 159±52 min and 34±9 min, respectively. This is comparable to our CPB time of 156 min and HCA time of 9 min. These techniques have allowed safe reoperations on patients who are considered as high surgical risk.
In conclusion, long-term follow-up is needed to fully evaluate the effectiveness and safety of percutaneous approach for closure of pseudoaneurysms. Open surgical repair entails higher risk in patients with multiple co-morbidities and poses technical challenges but remains the gold standard for treatment.