Engineering cord blood to improve engraftment after cord blood transplant
Introduction
Despite multiple advantages of umbilical cord blood (CB) transplant (CBT) (1,2), it is associated with delayed engraftment and immune reconstitution leading to a greater risk of infections and graft rejection as compared with bone marrow (BM) or peripheral blood (PB) grafts (3-6). For instance, after myeloablative conditioning, neutrophil engraftment is achieved in about 20–30 days with unmanipulated CBT as compared to 10–20 days after PB and 15–25 days after BM transplant (7-13). This is partly explained by lower numbers of total nucleated cells (TNCs) and CD34+ cells in a CB graft, which are typically only about 5–10% of the doses available for PB or BM stem cell transplant (SCT) (7-13). The minimum recommended TNC dose is 2.5×107–3×107/kg for 5–6/6 matched CBT, while higher numbers are needed for greater HLA-mismatch.
Several groups have shown that T cell immune reconstitution after double or single CBT (with or without serotherapy) is delayed (14,15) and this, along with the naivety of the infused CB T cells, correlates with an increased risk of viral reactivation or infection from latent and lytic viruses like cytomegalovirus (CMV), Epstein Barr virus (EBV) and adenovirus in the post-transplantation period (16-18). A comparison of immune recovery after double CBT and HLA-matched unrelated donor (MUD) revealed delay in recovery of naive (CD45RO) memory (CD45RO+) CD4+ T cells, CD8+ T cells, and regulatory CD4+ CD25+ T cells, which correlates with the increased viral infection in the early post transplantation period (15). A delayed time to recovery of thymopoiesis has been reported in recipients of UCBT compared with recipients of other graft sources (19).
With the increasing use of CB as alternative donor, efforts are being made to address the above limitations. One strategy to overcome this is the use of 2 partially HLA-matched CB units, which circumvents the limitation of low cell dose (9), but it still does not produce rapid engraftment as seen with PB and BM SCT. Another technique is co-infusion of one or two CB units along with CD34+-selected PB stem cells from HLA-haploidentical donor, known as the “Haplo-Cord” transplant (20-24). Two other methods to augment engraftment, which can be employed in the setting of either single or double unit CBT, include ex vivo expansion and/or ex vivo manipulation to improving BM homing ability of CB progenitor cells and immune modulation.
The purpose of this review is to describe the advances in CB engineering to improve CB engraftment and immune reconstitution.
Unique features of CB
It is important to acknowledge the quantitative and qualitative differences in the composition of UCB and hematopoietic stem cells (HSCs) from PB grafts prior to developing strategies for expansion (25). While UCB contains a higher concentration of HSC than adult PB, each unit contains one to two log lower total cell dose compared to BM and PBSC harvests (26). Furthermore, the vast majority of T cells within UCB are antigen-inexperienced naïve (CD45RA+), being less responsive to allogeneic stimulation, having reduced expression of transcription factors associated with T-cell activation [e.g., nuclear factor of activated T cells (NFAT)], and producing lower levels of effector cytokines compared to activated T cells from adult PB (27-29). The immaturity of UCB dendritic cells is also associated with lower antigen presenting activity, reduced expression of co-stimulatory molecules (CD80, CD86), reduced cytokine production [TNFα, interleukin (IL)-12], and an inherent ability to induce immune tolerance through peripheral expansion of regulatory T cells (Tregs) (30).
Ex vivo expansion of CB-derived stem cells
Multiple methods of CB expansion have been tested—varying remarkably from the starting cell population used for expansion (CD34+ versus CD133+ selected), the type of device used for cell extraction (Miltenyi CliniMacs or Nexell Isolex-300i), type of culture media (with or without serum), combination of cytokines [stem cell factor (SCF), granulocyte colony stimulating factor (G-CSF), granulocyte-macrophage colony-stimulating factor (GM-CSF), Fms-like tyrosine kinase 3 ligand (FLT-3L), IL-3, IL-6, IL-11, megakaryocyte growth and differentiation factor (MGDF) and thrombopoietin (TPO)], duration of culture (ranging from 1–10 weeks) and whether or not stromal support is used (31-41). In addition, the timing of thawing of the CB unit in context of the timing of transplant, and the proportion of CB unit used for expansion also varied considerably.
Static culture technique for CB stem cell expansion
One of the earliest clinical trials assessing the utility of ex vivo expanded CBT was reported by Shpall et al. (42) in the setting of single unit CBT in 37 patients (25 adults, 12 children), following myeloablative total body irradiation (TBI) or busulfan based conditioning. All patients received horse antithymocyte globulin (ATG) 30 mg/kg daily on days −5, −4, and −3. Graft versus host disease (GVHD) prophylaxis was provided with cyclosporine and prednisone. The study was conducted in two strata involving different methods. In the first stratum (n=25), the entire CB unit was thawed on day 0. A part of it (40–60%) was infused on day 0, while the rest was expanded ex vivo and infused on day +10. In the second stratum (n=12), only a fraction of the CB unit to be expanded was thawed (40–60%) on day −10. This portion was set in culture for expansion and infused on day 0 along with freshly thawed rest of the CB fraction. The technique of expansion was similar in both strata. CD34+ cells were selected from CB mononuclear cells (CBMC) with the Isolex 300-i device (Nexell, Irvine, CA, USA) and anti-CD34 antibody, which yielded only 30–45% of the initial thawed CD34+ cells. The purified cells were then cultured in a serum-free medium containing SCF, G-CSF and MGDF for 10 days. Although the culture resulted in a median of four-fold expansion of CD34+ count, yet the median CD34+ dose available for infusion in adult patients was only 0.89×105/kg (median TNC 0.79×107/kg) attributed to the initial significant cell loss from the selection technique. As a result, no improvement in engraftment was noted. The median time to neutrophil engraftment ranged from 26–31 days and that of platelet engraftment ranged from and 73–126 days, depending upon the stratum and the cohort involved. Further, the rates of acute grade III/IV acute GVHD (40%) and extensive chronic GVHD (63%) were particularly high despite no detectable T-cells in the CD34+ expanded population (42).
Continuous perfusion method
Shortly thereafter, investigators from the Duke University Medical Center reported another innovative CB expansion technique in 28 patients (median age 4.5 years; median weight 17 kg) with malignant or nonmalignant disorders who received single unit CBT after myeloablative TBI or busulfan based conditioning (43). All patients received ATG, cyclosporine plus methylprednisolone. The authors used an automated culture device, known as the Aastrom Replicell bioreactor (Aastrom Biosciences) where a computer generated system maintained the optimal culture conditions by continuously monitoring the biological and physiological culture environment and the bioreactor continuously perfused the culture with cytokines. The CB units were thawed on day 0, a majority of which was infused unmanipulated on the same day (median TNC 2.05×107/kg; median CD34+ 0.78×105/kg) while a small fraction (TNC, 1×108–2×108 total cells) was used for expansion and infused as a “boost” on day +12. The culture was supplemented with PIXY321 (a fusion protein of IL-3 and GM-CSF), FLT3-L and EPO. After 12 days of expansion, TNCs increased by a median of 2.4-fold, but the expansion of CD34+ fraction was rather feeble (median, 0.5-fold; range, 0.09–2.45). The median infused TNC dose of expanded cells was 2.05×107/kg and that of CD34+ was 0.10×105/kg. Three out of 22 patients failed to engraft while two died prior to engraftment. In rest of the evaluable patients, the median time to neutrophil engraftment was 22 days and that of platelets was 71 days. Grade II–IV acute GVHD occurred in 36% (8/22) (43).
Early progenitor cell (EPC) differentiation blockers
Despite modest expansion of cells in the trials mentioned above, failure to accelerate engraftment suggested that there might be potential loss of EPCs during the culture process resulting from differentiation of CD34+ cells to mature lineages (44). Therefore, methods to block in vitro differentiation of CD34+ cells in an attempt to expand EPCs were explored.
Copper chelators
Severe anemia and neutropenia are well-recognized signs of copper deficiency, resulting from ineffective erythropoiesis and granulopoiesis due to the arrest of progenitor cell maturation (45-47). Similar effects can be induced by using a high affinity copper chelator which decreases the intracellular copper content (48). In pre-clinical studies, the addition of polyamine copper chelator tetraethylenepentamine (TEPA; StemEx) to a culture containing cytokine cocktail of FLT-3L, IL-6, TPO and SCF inhibited the differentiation of EPCs (CD34+ CD38− or CD34+ Lin-) without affecting the proliferation of mature committed cells (CD34+ Lin+) (49), and resulted in substantial expansion of CD34+ (89-fold), CD34+ CD38− (30-fold) and colony forming units (CFUs; 172-fold) after 3 weeks (50). When tested in a phase I/II clinical trial (n=10), this technique did not lead to improvement in the engraftment of neutrophils (median, 30 days) after single unit CBT (51). Of note, methotrexate (5 mg/m2 on days +1, +3, +6 and +11) was used for GVHD prophylaxis, which may have delayed the engraftment (51). This method was then explored in a multi-center international trial including 101 adult patients (median age 37 years; median weight 68 kg) who underwent single unit CBT (52). Total median infused doses were 2.2×107 TNC/kg and 9.7×105 CD34+/kg. The median times to neutrophil engraftment (21 vs. 28 days, P<0.0001) and platelet engraftment (54 vs. 105 days, P=0.008) in the study group were remarkably faster than in the double unit CB transplant (DCBT) controls (n=295) from the Center for International Blood & Marrow Transplant Research (CIBMTR) and the Eurocord registries. Moreover, the study group had significantly superior overall survival at day 100 (84.2%) as compared to controls (74.6%), P=0.035. These resulted were presented at the American Society of Hematology meeting in 2013 (52); we are eagerly waiting for the complete results to be published.
Notch signaling
An innovative study from the Fred Hutchinson Cancer Research Center demonstrated that constitutive Notch signaling resulted in the development of immortalized, cytokine-dependent cell lines which were capable of generating either myeloid or lymphoid progeny after respective cytokine stimulation (53). In their subsequent study, CD34+ selected CB cells were transduced with an engineered Notch ligand (Delta1ext-IgG) and cultured for 16 days with IL-3, IL-6, TPO, SCF and FLT3-L, which lead to an average of 222-fold expansion of CD34+ cells as compared to 68-fold expansion of cells cultured with control IgG.
This led to the initiation of a phase-I clinical trial using this technique, the preliminary results of which were reported in 10 patients with acute leukemia with a median age of 27.5 years (range, 3 to 43) and median weight of 61.5 kg (range, 16 to 79). All patients underwent DCBT with one unmanipulated and one ex vivo expanded CB unit after receiving a myeloablative preparative regimen of Flu/Cy/TBI (fludarabine 75 mg/m2, cyclophosphamide 120 mg/kg and TBI 1,320 cGy). GVHD prophylaxis consisted of cyclosporine and mycophenolate mofetil (MMF). One CB unit was thawed 16 days before the transplant, selected for CD34+ cells and cultured as described above. On the day of transplant, the second CB unit was thawed and infused (unmanipulated), followed 4 hours later by the infusion the expanded unit. The culture resulted in an average fold expansion of TNCs by 562-fold (range 146–1,496) and CD34+ cells by 164-fold (range, 41–471). As a result of this enormous expansion, the infused CD34+ cell dose from the expanded unit (average, 60×105/kg; range, 9.3×105–130×105) was significantly higher as compared to that from the unmanipulated unit (average, 2.4×105/kg; range, 0.6×106–5.4×106). The expanded graft contained no mature T cells. One patient experienced primary graft rejection. The median time to neutrophil engraftment was 16 days (range, 7–34 days) as compared to 26 days (range, 16–48 days; P=0.002) in controls (n=20). All evaluable patients developed grade II acute GVHD, except one who had grade III acute GVHD. Limited chronic GVHD occurred in 3 patients and nobody developed extensive chronic GVHD. With a median follow-up 354 days, 70% were alive in complete remission with sustained engraftment. Among 8 evaluable patients, 50% had engraftment from the expanded cord at the time of engraftment while the rest engrafted from the unmanipulated unit. Interestingly, two patients were noted to have long-term persistence of the expanded cells till day 180 and day 240, but not after one year, after which the unexpanded cord completely contributed to engraftment (54).
Nicotinamide (NAM)
Another method of blocking in vitro differentiation of EPCs is the use of a vitamin B3 analogue—NAM, which inhibits several key enzymes that require NAD+, including the sirtuin family of histone/protein deacetylases (such as SIRT1) that regulate several biological pathways involving cell adhesion, migration, proliferation and differentiation (55-59). In preclinical studies, ex vivo treatment of CB CD34+ cells with NAM and cytokines led to their robust expansion (60,61).
The efficacy of NAM-expanded CBT was then tested in a clinical trial involving 11 adult patients (median age 45 years, median weight 83 kg) in the setting of DCBT after a myeloablative preparative regimen of Flu/Cy/TBI or Flu/TBI. GVHD prophylaxis was provided by tacrolimus and MMF. One of the CB units was thawed, selected for CD133+ cells and expanded ex vivo for 3 weeks with SCF, TPO, IL-6 and FLT3-L. The negative fraction was re-cryopreserved, and later re-thawed and infused along with the expanded fraction and a second unmanipulated CB unit. The culture resulted in a median 486-fold (range, 171–643) expansion of TNCs and 72-fold (range, 16–186) expansion of CD34+ cells. The median infused TNCs of unmanipulated unit was 2.3×107/kg and the median infused CD34+ dose was 0.7×105/kg. When combined with CD133− fraction, the infused TNC dose of the expanded unit was 3.1×107 kg and the infused CD34+ dose was 3.5×105/kg. The median time to neutrophil engraftment was significantly faster in patients transplanted with the expanded CB unit (13 days) as compared with a historical cohort of double CBT (DCBT) (25 days), P<0.001. There was no difference in the median time to platelet engraftment (33 vs. 37 days, P=0.085). Half of the patients engrafted from the expanded unit, one-fourth engrafted with the unexpanded unit and the rest had dual-chimerism from both the units. The expanded CB unit provided long-term engraftment in 8 of 10 evaluable patients, which remained stable for up to 36 months. Acute grade II GVHD occurred in 5 out of 11 patients; there were no cases of grades III–IV acute GVHD and 2 patients developed chronic GVHD (62).
Stem regenin-1 (SR1)
Most recently, Wagner et al. reported the results of CB expansion using SR1 (63), which directly binds to and inhibits aryl hydrocarbon receptor (which promotes differentiation of EPCs) (64,65). In this study, 17 patients (median age 29.9 years, median weight 87.1 kg) underwent DCBT after a myeloablative Flu/Cy/TBI conditioning. On day −15, CD34+ cells were enriched from the lower cell-dosed CB unit and placed in culture for expansion in the presence of SCF, FLT-3L, TPO, IL-6, and SR1. The negative fraction (CD34− population) was re-cryopreserved. On day 0, the unmanipulated unit was thawed and infused, followed 4 hours later by the infusion of SR1-expanded CB unit and then the infusion of CD34− population 4–24 hours later. The culture resulted in a median 854-fold expansion of TNCs and 330-fold expansion of CD34+ cells, providing massive total cell doses for infusion (TNC: median, 7; range, 3×107–17×107/kg and CD34+: median, 182; range, 23×105–485×105/kg). There were no graft failures. Engraftment of neutrophils (median 15 vs. 24 days) and platelets (median 49 vs. 89 days, P=0.001) was significantly faster in the study group than in the historical controls (n=111). The expanded cord provided engraftment in 65% (11/17) of patients while the remaining patients engrafted from the unmanipulated cord. Patients who engrafted from the expanded cord had durable myeloid engraftment (median follow-up, 272 days) with neutrophil engraftment occurring at a median of 11 days as compared to 23 days (range, 14–30 days) in those who engrafted from the unmanipulated cord. This resulted in a significant reduction in the length of hospitalization in the SR1-expanded population (median 30 versus 46 days) as compared to the controls, P<0.001. Other outcomes, including grade II–IV, grade III–IV acute GVHD, transplant-related mortality and overall survival were similar in both the groups.
Mesenchymal stem cell (MSC) supported expansion
Another technique of ex vivo expansion simulates the in vivo growth environment of EPCs by using MSCs during culture, which provides a “niche” for hematopoiesis by producing cytokines and other proteins that regulate hematopoietic cell proliferation as well as homing (66). Co-culturing with MSCs also prevents the initial cell loss that occurs during the selection process, as it does not require purification of either CD34+ or CD133+ cells (67). Moreover, clinical grade “off the shelf” MSC precursor cells, such as mesoblast (Mesoblast Limited, Melbourne, Australia), are now available where MSCs are isolated using a Stro monoclonal antibody (68), therefore making them promptly available.
Using this technique a prominent trial involving 31 patients was conducted by de Lima et al. at MD Anderson Cancer Center (MDACC) (69), where one unit was expanded ex vivo for two weeks and infused concurrently with the unexpanded unit. All patients underwent myeloablative conditioning with Flu/Mel/Thiotepa and received rabbit ATG (3 mg/kg), tacrolimus and MMF. The source of MSCs was a haploidentical donor in the first 7 patients, while rest of the patients received “off the shelf” “third party” MSCs (Mesoblast Limited, Melbourne, Australia). The smaller CB unit was thawed 2 weeks prior to transplant and co-cultured with MSCs along with SCF, FLT-3L, TPO and G-CSF. The culture resulted in significant increases in CD133+CD33+ and CD34+CD38− cell populations, suggesting expansion of EPCs. After 2 weeks of culture, TNC, CD34+, and CFU-C populations increased by 12.2-, 30.1- and 17.5-fold, respectively. Consequently, the median infused TNCs (5.84×107 versus 2.28×107/kg) and CD34+ cells (9.5×105 versus 3.8×105/kg) were higher in the expanded unit compared with the unexpanded CB unit. The unmanipulated unit was thawed on day 0, washed, and infused followed by infusion of the expanded UCB unit. As compared with 80 controls patients from the CIBMTR, neutrophil engraftment was significantly faster (median 15 vs. 24 days, P<0.001) in the study group. The median time to platelet engraftment was reduced by one week (42 vs. 49 days, P=0.03) in the study group. The expanded CB unit was present in only 13% of the patients at 6 months while long term engraftment beyond 1-year was provided solely by the unmanipulated unit. The cumulative incidence of grade II–IV acute GVHD (42%), grade III–IV acute GVHD (13%), chronic GVHD (45%) was similar as in the CIBMTR or institutional controls.
Improving homing capacity of CB progenitor cells
Apart from boosting the progenitor cell content of a graft, another mutually non-exclusive technique to augment engraftment involves increasing the capacity of CB progenitor cells to home to BM. Three published trials explored this concept, two of which showed dramatic improvement in engraftment without affecting the cell dose.
Prostaglandin E2 (PGE2) analog
PGE2 stimulates the growth and homing potential of multilineage progenitor cells, which is postulated to be mediated via up-regulation of survivin (apoptosis-inhibiting protein) cyclin D1 (proliferation gene) and the chemokine receptor CXCR4 (which promotes adhesion and homing) (70).
In a phase-I clinical trial, the group from Dana Farber (71) evaluated the safety and efficacy of a PGE2 derivative (dmPGE2) in 21 patients who underwent DCBT after Flu/Mel (fludarabine 180 mg/m2 and melphalan 100 mg/m2) conditioning. All patients received ATG (4 mg/kg), tacrolimus and sirolimus. The study included two separate cohorts which differed in the incubation techniques. In cohort 1 (n=9), one of the CB units was incubated with 10 mM of dmPGE2 (fate therapeutics) for 1 hour at 4 °C. This method was changed after the authors noticed 2 cases of primary graft failure and no improvement in the rates of neutrophil recovery (median 24 days). After conducting a comprehensive genome-wide expression analysis by varying the culture techniques, the authors found that 2-hour incubation with prostaglandin at 37 °C was required to induce the maximal activation of the prostaglandin pathway. Therefore, for the second cohort (n=12), they incubated one CB unit in the same solution as above but for 2 hours at 37 °C. The smaller of the units was treated ex vivo in 6 out of 9 patients in cohort 1, while the larger unit underwent treatment in rest of the patients in cohort 1 and all of the patients in cohort 2. Both the units were thawed on day 0 and infused within 4 hours of each other (larger followed by the smaller unit). The median ages in cohort 1 and 2 were 43 (range, 29–64) and 57.5 (range, 19–66) years, respectively, and the median weights were 73.8 (range, 44.7–126) kg and 78.7 (48.7–149.6) kg, respectively. There were no cases of primary graft failure in cohort 2, which also experienced significantly faster neutrophil engraftment (median 17.5 days; range, 14–31 days), as compared with the controls (21 day), P=0.045. The median time to platelet engraftment was 43 days (range, 26–60 days). In addition, 83% (10/12) of patients had 100% chimerism from the treated CB unit, which provided sustained engraftment in some patients for up to 27 months. Grade I acute GVHD was seen in 3 patients, 2 patients had skin only grade II acute GVHD and 1 patient had skin only chronic GVHD (71).
Fucosylation
Another study exploited the concept of homing mediated through selectins and their ligands. It is know that successful homing of the transplanted cells results from a series of interactions between adhesion molecule receptors (E- or P-selectins) on vascular endothelial cells and selectin ligands on hematopoietic cells. One of the major shortcomings of CB progenitor cells is that they have an impaired capacity to home to BM, which is attributed to poor fucosylation of their selectin ligands, such as P-selectin glycoprotein ligand-1 (PSGL-1) (72). Treatment of human CB CD34+ cells with fucosyltransferase (FT)-VI and GDP-fucose improves their homing capacity and results in faster engraftment in mouse models (73).
The results of a phase I clinical trial at the MDACC including 22 adult patients (median age 42 years; median weight 79 kg) were recently reported (74). All patients received DCBT after either myeloablative conditioning with Bu/Flu/Clo/TBI (busulfan AUC 16,000 µmol/min, fludarabine 40 mg/m2, clofarabine 120 mg/m2 and TBI 2 Gy), or Flu/Mel (fludarabine 160 mg/m2 and melphalan 140 mg/m2). All patients received rabbit ATG (3 mg/kg), tacrolimus and MMF. On the day of transplant, the CB unit with the highest TNC dose was thawed and infused unmanipulated, while the smaller unit was thawed, washed and treated ex vivo at 37 °C for 30 minutes with FT-VI, GDP b-fucose, and MnCl2. The fucosylated cells were then washed and infused. The median infused TNC dose of the fucosylated CB unit was 1.75×107/kg and that of unmanipulated unit was 2.59×107/kg. The median infused CD34+ doses were 0.92×105/kg in the fucosylated unit and 1.75×105/kg in the unmanipulated unit. One patient died of sepsis on day 23 without engraftment and one developed secondary graft failure after initial engraftment on day 14. Among remaining patients, the median time to engraftment of neutrophils (17 vs. 26 days, P=0.0023) and platelets (35 vs. 45 days, P=0.05) was significantly shorter than in the historical controls. All evaluable patients had 100% chimerism on day +30–40% from the fucosylated unit, 40% from the unmanipulated unit and 20% had dual chimerism from both units. There were no differences in neutrophil or platelet engraftment among those who engrafted from fucosylated versus unmanipulated unit. The cumulative incidence of grade II–IV acute GVHD was 40.9%, grade III–IV was 9.1% and chronic GVHD 5%, which were similar to that in controls (74).
DPPIV inhibitor
Another study examined the efficacy of an oral dipeptidyl peptidase-4 (DPPIV) inhibitor—sitagliptin, which is a commonly used antidiabetic drug. DPPIV/CD26 is a membrane bound peptidase that inhibits the migratory potential of CD34+ cells by cleaving CXCL12/SDF-1α (stromal cell-derived factor 1) at its N-terminal (75). An active form of DPPIV/CD26 is present on a small sub-set of CB CD34+ cells. Inhibition of DPPIV activity using a specific inhibitor (diprotin-A) significantly increased the homing capacity of CD34+ /CD26+ cells (75) as well as long-term engraftment of Sca-1+ lin- BM donor cells in mouse models (76).
A clinical trial assessed its safety and efficacy in 24 patients with hematological malignancies who received myeloablative conditioning with TBI (13.2 Gy) and cyclophosphamide (120 mg/kg) followed by single unit CBT. All patients received sitagliptin, 600 mg once daily from day −1 to day +2. The median infused TNC was 2.3×107/kg and the median infused CD34+ dose was 1×105/kg. The median time to neutrophil engraftment was 21 (range, 13–50) days and that of platelet engraftment was 77 (range, 48–220) days, which are not improved over reported results of unmanipulated CBT (77,78).
Expansion of CB derived T cells
Expanding T cells for donor lymphocyte infusion (DLI)
Given the limited cell number and non-availability of donor, the use of DLI to boost post-transplant immunity to prevent infections, treat mixed chimerism and disease relapse is currently restricted to pre-clinical and early phase trials. Many groups have investigated the possibility of ex vivo expansion of CB T cells. Investigators from the Karolinksa demonstrated the feasibility of this approach by using CD3/CD28 bead in presence of IL-2 to expand CB T cells with a yield of 148×106, starting with as few as 1.5×106 CD3+ cells (79). Another group used a similar approach but with additional use of IL-7 in culture to improve cell survival and function (80). This system allowed for expansion of poly-specific CD3+ T cells facilitating a DLI dose of 1×106/kg recipient body weight (81). In a pilot phase I study, four patients received a CB-derived DLI at a median 87 days (range, 48–137 days) after UCB transplantation (81). There were no infusion-related toxicities and there was no clinically GVHD arising as consequence of the DLI. In another phase 1 trial by Hexner et al., patients with CB frozen in 2 fractions received ex vivo expanded CB T cells at 1×105 cells/kg (82). There was evidence of early T cell trafficking and supraphysiological levels of cytokines relevant to engraftment. However, the study was stopped early due to grade 3 GVHD in 2/4 patients. Clinical activity of CB derived DLI thus awaits larger study (Table 1).
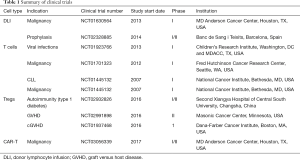
Full table
Virus directed T cells
Ex vivo expanded virus specific T cells (VSTs)
T cell immune reconstitution is essential to speed up the recover from viral infections. Adoptive T cell therapy using donor-derived ex vivo expanded T cells has emerged as an effective strategy in preventing and treating viral infections (83-85). Simplified methods for rapid production of multi-VSTs from seropositive individuals have been validated and used for prophylaxis and treatment in the post-transplant setting (86-88). There are however a few obstacles to extend this approach to the CB setting. These include: (I) the limited numbers of CB T-cells available for manipulation; and (II) the naivety of CB T cells. Hence, the development of virus-protective T cell therapy for patients undergoing CBT requires the priming and extensive expansion of naive T cells rather than the more limited and simple direct expansion of pre-existing VST populations from virus-experienced donors. As a result, the manufacture of CB-derived multi-VSTs requires manufacturing times of 10+ weeks (84).
Further, CB T-cells have lower cytotoxic activity and higher activation-induced cell death than PB T-cells. These limitations have prevented the production of CB derived VSTs in sufficient numbers for clinical use. Because of these challenges, only a few reports document the generation of antigen-specific T cells from CB. Sun et al. first reported the ability to generate EBV-specific CD4+ T cells using EBV-transformed B-cells, or lymphoblastoid cell lines (LCL) (89). Park et al. then reported the ability to generate CMV-pp65-specific T cells from CB by using CMV-immune complex-loaded DCs, CMV lysate, and IL-12 and IL-7 (90). Subsequently, our group demonstrated the ability to produce CMV, EBV and adenovirus VSTs from 20% fraction of CB unit using DCs transduced with Ad5f35pp65-vector in presence of IL-7, IL-12 and IL-15 (84). A clinical trial using CB-derived multi-VSTs for the prevention and treatment of viral infection after CBT was started and completed at BCM. (NCT01017705). On this phase I study 13 patients received VSTs generated from the 20% fraction of a fractionated CB unit. VSTs were infused to 13 patients from 63–443 days post CBT at doses of 5×106 to 2.5×107/m2 (88,91,92). No infusion-related toxicities or GvHD was observed. Within 2 weeks of VST infusion, all patients had detectable virus-specific T cells in their PB that persisted up to 1 year post T-cell infusion (88,92). Further, using TCR deep sequencing we have shown that T cell clones present in the VST products of all patients, but not in their PB pre infusion, expand up to 14 fold post infusion. Six patients remain free of CMV, EBV, and Adv reactivation. The patients who developed reactivation had spontaneous resolution or responded to additional dose of VSTs (91).
To avoid using an adenoviral vector, we then manufactured CB-derived multivirus-specific T cell products using antigen presenting cells (APC) pulsed with clinical grade viral pepmixes spanning Adv (Hexon and Penton), CMV (IE1 and pp65), EBV (LMP2, EBNA1) in the presence of the cytokines. Four patients have received VSTs on this phase 1 study on this phase 1 trial (NCT01923766). By day 45, three patients were virus free and one patient had reduced CMV levels in the detectable but not quantifiable range (88,93). Interestingly, epitope mapping showed that the immunodominant CMV-pp65 epitopes recognized by these T cells differed from T cells manufactured from CMV seropositive adult donors, but in preliminary studies still appeared to confer protection (92).
The above studies however still have the limitations of the lengthy manufacturing time of over 3 months (84). Strategies to simplify the manufacture method further to reduce the length of manufacture time to 4 weeks and expand the repertoire to multiple viruses such as BKV are underway and hold promise (94).
TCR-gene modification
An alternative approach to generate virus directed T cells in the naive donor is to transduce T cells with a TCR with known specificity to an HLA restricted epitope identified within an immunogenic viral antigen (95,96). Although this strategy offers a novel, rapid method to generate VSTs from naive donors, it is costly and imposes additional regulatory requirements of gene transfer. In addition, the strategy is HLA restricted and targeting a single viral epitope increases the risk of viral immune escape. However, a clinical trial is currently underway in the United Kingdom evaluating T cells transduced with a retroviral vector expressing a CMV-specific TCR for high-risk patients after HSCT (Morris et al.) (97).
Viral epitope specific T cells
Another group demonstrated that CD34+CD38−/low UCB-derived HSCs can be differentiated to early T cells using surface-immobilized Notch ligands, which when stimulated with HLA-A*0201 tetramers for CMV and Influenza-A produced functional and viral specific CD8+ T cells (98). This method avoids the co-culture with stromal cells as well as retroviral TCR receptors and is another promising method with scalable potential for adoptive T cell transfer. However, the HLA restriction remains a limitation both for patient eligibility and for the potential for viral immune escape.
HIV-specific T cells
The success of the Berlin patient has stimulated interest in allogeneic-VST therapy for the treatment of HIV (99). In a recent report, T cells specific for multiple HIV epitopes, irrespective of donor HLA type, were expanded from seronegative adult donors using DCs pulsed with pepmixes for HIV antigens (100). These T cells suppressed viral replication compared with unexpanded CD8 T cells, and CMV and EBV-specific T cells derived from the same HIV-seronegative donors. This holds promise in the CB setting and HIV-specific T cells manufactured this way are currently studies utilizing donor-derived HIV-specific T cells after allogeneic HSCT are being planned (97). Moreover, the group at Children’s National is currently exploring the potential expanding HIV-specific T cells from CB using a non-HLA restricted GMP compliant approach (101).
Tumor-directed T cells
Ex vivo expanded tumor specific T cells
The use of CB-derived antigen-specific T cells, however, is not limited to virus-specific T cells, as highlighted in a study by Merindol et al. (102). They expanded Melan-A/MART-1-specific T cells from CB by culturing CBMC with HLA-A2+ LCL pulsed with the HLA-A2-restricted modified Melan-A A27L peptide. The resulting cytotoxic T lymphocyte products exhibited less alloreactivity than T cells generated using the same method derived from the PB of adult donors. PR-1 is a leukemia associated antigen found in PB of patients with myeloid leukemia. St John et al. reported an increased frequency of PR-1 specific CTLs in CB (0.14% of CD8+ T cells), which is 45 times higher than found in PB of healthy adult donor (103). They were then able to expand PR-1 specific T cells from CBMC using peptide pulsed gene modified K562s as artificial APCs that expressed effector memory phenotype and were able to lyse PR-1 specific targets in vitro (103).
The use of a single tumor associated antigen (TAA) peptide limits its application to only well characterized peptide sequences, which in most cases are HLA restricted. Weber et al. developed a strategy to rapidly expand multi-TAA CD4+ and CD8+ cytotoxic T lymphocytes (TAA-T) from healthy adult donors and CB, regardless of their HLA-type (104). They used peptide libraries of 15 mer peptides overlapping by 11 amino acids spanning the whole sequence of 5 target antigens Wilm tumor gene1 (WT1), Melanoma associated antigen A3 (MAGE-A3), proteinase 3 (Pr3), human neutrophil elastase (NE) and preferentially expressed antigen in melanoma (PRAME). The TAA-T showed broad reactivity against a multiple target antigens, was polyfunctional and killed primary leukemia blasts in vitro. CB derived TAA-T showed different HLA-restricted epitope specificity compared with adult donor-TAA-T, which will need to be explored in future studies. There was no antigen competition observed and thus other TAA can be easily incorporated to broaden the repertoire of TAA-specific T cells.
Chimeric antigen receptor (CAR) transduced T cells
CAR modified T cells targeting tumor antigens have demonstrated remarkable success in many hematologic malignancies (5,105-109). This approach has also been translated in the CB setting. Micklethwaite et al. demonstrated the feasibility of producing a single product that was virus specific (through native endogenous TCR) and tumor specific (through co-stimulatory molecules from a CD19+ CAR) as an effective antiviral and anti-leukemia therapy after CBT for patients with high risk B-ALL (110). CD19+ CAR T cells were produced by transducing the VSTs with CD19 retrovirus supernatant at the time of the third stimulation of the VSTs. The resultant CAR-T cells retained viral specificity and lyse CD19 expressing leukemia targets. The same group has also developed a TCR-like antibody that recognizes PR-1/HLA2 complex on acute myeloid leukemia cells and generated a second generation CAR using CBMC (111). In order to avoid using viral supernatant for transduction, the group at MD Anderson used electro-transfer of non-viral plasmids of the gene of interest (112). They adapted a Sleeping Beauty transposon and transposase along with artificial APCS to scale up the manufacture of CD19-directed CAR T cells from CBMCs. After 28 days of continuous culture, they were able to generate 1010 genetically modified T cells for clinical application (112,113).
The group at Memorial Sloan Kettering expanded CB derived-T cells in presence of IL-12 and IL-15 (114). These CB T cells were then modified to both express the CD19-specific CAR, 1928z, and secrete IL-12. The 1928z/IL-12 CB T cells retained a central memory-effector phenotype and had increased antitumor efficacy in vitro.
CB derived Tregs
Tregs are a lineage of T cells possessing the ability to inhibit other T-cell responses. Naturally occurring Tregs are CD4+, CD25+, express the forkhead box protein 3 (FoxP3) and represent 1–5% of circulating CD4+ T cells (115). Adoptive transfer of donor Tregs has been explored as a strategy to decrease the severity of acute GVHD and as treatment for chronic GVHD (116-118). However, the clinical translation of this approach has been limited even in the PB setting due to the large volume of blood required and the difficulty of isolating and expanding purified Tregs in numbers sufficient for clinical use. Although this problem is exaggerated in the CB setting, CB derived Tregs have been expanded using anti-CD3 and anto-CD28 microbeads in presence of exogenous IL-2 (119,120). The resultant Tregs were 4–10 times better than Treg from PB, allowing a 200–1,000-fold expansion in less than 3 weeks that could suppress effector T cells at ratios as low as 1 CB Treg to 3 effector T cells. CB-derived Treg using this approach have been used clinically by the Minnesota group (121,122), who have transferred HLA partially matched Treg to 19 transplant recipients. Results from this study appear encouraging, as evidenced by a delayed time to GVHD (compared with historical controls) and an increase in circulating Treg (122). Subsequent analysis of this cohort found an increased risk of viral infections within 30 days of the infusion; however the risk did not affect long-term survival and risk for later viral infections (121). Recently, the same group evaluated the safety of infusing Tregs expanded in cultures stimulated with K562 cells modified to express the high-affinity Fc receptor (CD64) and CD86, the natural ligand of CD28 (KT64/86) (123). Eleven patients were treated with Treg doses from 3×106–100×106 Treg/kg. There were no dose-limiting infusional toxicities and the incidence of grade II–IV acute GVHD at 100 days was 9% (95% CI, 0–25) vs. 45% (95% CI, 24–67) in contemporary controls. Chronic GVHD at 1 year was zero in Tregs and 14% in controls (123).
Future directions
The techniques for ex vivo expansion have progressed dramatically over time—reforming from the traditional method of cytokine-only static culture to automated continuous perfusion, and then focusing on expansion of EPCs by blocking their ex vivo differentiation using a variety of agents, such as copper chelators, NAM, SR1 or by targeting Notch signaling. However, all of these approaches require several days (up to 3 weeks) of culture before transplant, which adds to the cost and labor. Moreover, some patients may need to proceed to transplant immediately, in which case waiting for 3 weeks may not be ideal. Furthermore, some of these methods which produce enormous expansion of CD34+ cells inevitably lead to a relative state of T cell depletion in the graft—the consequence of which is unclear at this time. Furthermore, all of the current techniques can only be utilized in highly specialized centers, which limits their wider application.
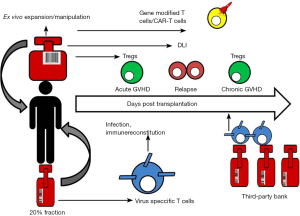
Given the large global inventories of frozen CB units, the development of CB-derived T cell product also provides a novel platform for third party use when adult donors are not readily available (Figure 1). Adoptive immunotherapy using banked VSTs partially HLA-matched donors has shown considerable success in treating EBV related lymphoma and EBV, adenovirus and CMV infections (124-126). The ready availability of clinical grade frozen CB units that are already screened and HLA typed makes CB derived VSTs an attractive and cost-saving option. Given the minimal HLA disparity, third-party VSTs can be used in non-HSCT setting for oncology patients or for those with primary immune deficiencies. Furthermore, because human leukocyte antigen matching is not required, allogeneic UCB Treg may be a useful strategy for prevention of organ rejection and autoimmune diseases (127). For therapeutic use, a banking system for UCB Treg would be able to greatly reduce cost and time to transplant. Mckenna et al. have optimized a convenient banking protocol to thaw Tregs that have been frozen down at the end of their first stimulation and restimulate just prior to infusion (127).
Conclusions
Over the past two decades, tremendous progress has been made to improve the outcomes of CBT by strategies focused on early engraftment, graft engineering and ex vivo expansion of hematopoietic progenitors and immune system cells. Several methods for ex vivo expansion of CB derived T cells for immune modulation have been validated and slowly being brought to the clinic. These strategies hold promise to restore improve outcomes after CBT by enhancing antiviral immunity, preventing GVHD and producing lasting anti-tumor activity.
Acknowledgements
None.
Footnote
Conflicts of Interest: The authors have no conflicts of interest to declare.
References
- Munoz J, Shah N, Rezvani K, et al. Concise review: umbilical cord blood transplantation: past, present, and future. Stem Cells Transl Med 2014;3:1435-43. [Crossref] [PubMed]
- Oran B, Shpall E. Umbilical cord blood transplantation: a maturing technology. Hematology Am Soc Hematol Educ Program 2012;2012:215-22. [PubMed]
- Eapen M, Rocha V, Sanz G, et al. Effect of graft source on unrelated donor haemopoietic stem-cell transplantation in adults with acute leukaemia: a retrospective analysis. Lancet Oncol 2010;11:653-60. [Crossref] [PubMed]
- Jacobson CA, Turki AT, McDonough SM, et al. Immune reconstitution after double umbilical cord blood stem cell transplantation: comparison with unrelated peripheral blood stem cell transplantation. Biol Blood Marrow Transplant 2012;18:565-74. [Crossref] [PubMed]
- Majhail NS, Brunstein CG, Tomblyn M, et al. Reduced-intensity allogeneic transplant in patients older than 55 years: unrelated umbilical cord blood is safe and effective for patients without a matched related donor. Biol Blood Marrow Transplant 2008;14:282-9. [Crossref] [PubMed]
- Brunstein CG, Gutman JA, Weisdorf DJ, et al. Allogeneic hematopoietic cell transplantation for hematologic malignancy: relative risks and benefits of double umbilical cord blood. Blood 2010;116:4693-9. [Crossref] [PubMed]
- Laughlin MJ, Eapen M, Rubinstein P, et al. Outcomes after transplantation of cord blood or bone marrow from unrelated donors in adults with leukemia. N Engl J Med 2004;351:2265-75. [Crossref] [PubMed]
- Rocha V, Labopin M, Sanz G, et al. Transplants of umbilical-cord blood or bone marrow from unrelated donors in adults with acute leukemia. N Engl J Med 2004;351:2276-85. [Crossref] [PubMed]
- Barker JN, Weisdorf DJ, DeFor TE, et al. Transplantation of 2 partially HLA-matched umbilical cord blood units to enhance engraftment in adults with hematologic malignancy. Blood 2005;105:1343-7. [Crossref] [PubMed]
- Cornetta K, Laughlin M, Carter S, et al. Umbilical cord blood transplantation in adults: results of the prospective Cord Blood Transplantation (COBLT). Biol Blood Marrow Transplant 2005;11:149-60. [Crossref] [PubMed]
- Takahashi S, Ooi J, Tomonari A, et al. Comparative single-institute analysis of cord blood transplantation from unrelated donors with bone marrow or peripheral blood stem-cell transplants from related donors in adult patients with hematologic malignancies after myeloablative conditioning regimen. Blood 2007;109:1322-30. [Crossref] [PubMed]
- Raiola AM, Dominietto A, di Grazia C, et al. Unmanipulated haploidentical transplants compared with other alternative donors and matched sibling grafts. Biol Blood Marrow Transplant 2014;20:1573-9. [Crossref] [PubMed]
- Barker JN, Fei M, Karanes C, et al. Results of a prospective multicentre myeloablative double-unit cord blood transplantation trial in adult patients with acute leukaemia and myelodysplasia. Br J Haematol 2015;168:405-12. [Crossref] [PubMed]
- Lucchini G, Perales MA, Veys P. Immune reconstitution after cord blood transplantation: peculiarities, clinical implications and management strategies. Cytotherapy 2015;17:711-22. [Crossref] [PubMed]
- Saliba RM, Rezvani K, Leen A, et al. General and Virus-Specific Immune Cell Reconstitution after Double Cord Blood Transplantation. Biol Blood Marrow Transplant 2015;21:1284-90. [Crossref] [PubMed]
- Ballen K, Woo Ahn K, Chen M, et al. Infection Rates among Acute Leukemia Patients Receiving Alternative Donor Hematopoietic Cell Transplantation. Biol Blood Marrow Transplant 2016;22:1636-45. [Crossref] [PubMed]
- Escalon MP, Komanduri KV. Cord blood transplantation: evolving strategies to improve engraftment and immune reconstitution. Curr Opin Oncol 2010;22:122-9. [Crossref] [PubMed]
- Geyer MB, Jacobson JS, Freedman J, et al. A comparison of immune reconstitution and graft-versus-host disease following myeloablative conditioning versus reduced toxicity conditioning and umbilical cord blood transplantation in paediatric recipients. Br J Haematol 2011;155:218-34. [Crossref] [PubMed]
- Komanduri KV, St John LS, de Lima M, et al. Delayed immune reconstitution after cord blood transplantation is characterized by impaired thymopoiesis and late memory T-cell skewing. Blood 2007;110:4543-51. [Crossref] [PubMed]
- Kwon M, Bautista G, Balsalobre P, et al. Haplo-Cord transplantation compared to haploidentical transplantation with post-transplant cyclophosphamide in patients with AML. Bone Marrow Transplant 2017. [Epub ahead of print]. [Crossref] [PubMed]
- Kwon M, Bautista G, Balsalobre P, et al. Haplo-cord transplantation using CD34+ cells from a third-party donor to speed engraftment in high-risk patients with hematologic disorders. Biol Blood Marrow Transplant 2014;20:2015-22. [Crossref] [PubMed]
- Liu H, Rich ES, Godley L, et al. Reduced-intensity conditioning with combined haploidentical and cord blood transplantation results in rapid engraftment, low GVHD, and durable remissions. Blood 2011;118:6438-45. [Crossref] [PubMed]
- Fernandez MN, Regidor C, Cabrera R, et al. Unrelated umbilical cord blood transplants in adults: Early recovery of neutrophils by supportive co-transplantation of a low number of highly purified peripheral blood CD34+ cells from an HLA-haploidentical donor. Exp Hematol 2003;31:535-44. [Crossref] [PubMed]
- Barker JN, Ponce DM, Dahi PB, et al. Double-Unit Cord Blood (CB) Transplantation Combined With Haplo-Identical CD34+ Cell-Selected PBSC Results In 100% CB Engraftment With Enhanced Myeloid Recovery. Blood 2013;122:298. [PubMed]
- Rocha V, Broxmeyer HE. New approaches for improving engraftment after cord blood transplantation. Biol Blood Marrow Transplant 2010;16:S126-32. [Crossref] [PubMed]
- Kim DK, Fujiki Y, Fukushima T, et al. Comparison of hematopoietic activities of human bone marrow and umbilical cord blood CD34 positive and negative cells. Stem Cells 1999;17:286-94. [Crossref] [PubMed]
- Kadereit S, Mohammad SF, Miller RE, et al. Reduced NFAT1 protein expression in human umbilical cord blood T lymphocytes. Blood 1999;94:3101-7. [PubMed]
- Barrios C, Brandt C, Berney M, et al. Partial correction of the TH2/TH1 imbalance in neonatal murine responses to vaccine antigens through selective adjuvant effects. Eur J Immunol 1996;26:2666-70. [Crossref] [PubMed]
- Delespesse G, Yang LP, Ohshima Y, et al. Maturation of human neonatal CD4+ and CD8+ T lymphocytes into Th1/Th2 effectors. Vaccine 1998;16:1415-9. [Crossref] [PubMed]
- Drohan L, Harding JJ, Holm B, et al. Selective developmental defects of cord blood antigen-presenting cell subsets. Hum Immunol 2004;65:1356-69. [Crossref] [PubMed]
- Lazzari L, Lucchi S, Porretti L, et al. Comparison of different serum-free media for ex vivo expansion of HPCs from cord blood using thrombopoietin, Flt-3 ligand, IL-6, and IL-11. Transfusion 2001;41:718-9. [Crossref] [PubMed]
- Lazzari L, Lucchi S, Rebulla P, et al. Long-term expansion and maintenance of cord blood haematopoietic stem cells using thrombopoietin, Flt3-ligand, interleukin (IL)-6 and IL-11 in a serum-free and stroma-free culture system. Br J Haematol 2001;112:397-404. [Crossref] [PubMed]
- McNiece I, Kubegov D, Kerzic P, et al. Increased expansion and differentiation of cord blood products using a two-step expansion culture. Exp Hematol 2000;28:1181-6. [Crossref] [PubMed]
- Mohamed AA, Ibrahim AM, El-Masry MW, et al. Ex vivo expansion of stem cells: defining optimum conditions using various cytokines. Lab Hematol 2006;12:86-93. [Crossref] [PubMed]
- Piacibello W, Sanavio F, Garetto L, et al. Differential growth factor requirement of primitive cord blood hematopoietic stem cell for self-renewal and amplification vs proliferation and differentiation. Leukemia 1998;12:718-27. [Crossref] [PubMed]
- Yao CL, Chu IM, Hsieh TB, et al. A systematic strategy to optimize ex vivo expansion medium for human hematopoietic stem cells derived from umbilical cord blood mononuclear cells. Exp Hematol 2004;32:720-7. [Crossref] [PubMed]
- Yao CL, Feng YH, Lin XZ, et al. Characterization of serum-free ex vivo-expanded hematopoietic stem cells derived from human umbilical cord blood CD133(+) cells. Stem Cells Dev 2006;15:70-8. [Crossref] [PubMed]
- Madkaikar M, Ghosh K, Gupta M, et al. Ex vivo expansion of umbilical cord blood stem cells using different combinations of cytokines and stromal cells. Acta Haematol 2007;118:153-9. [Crossref] [PubMed]
- Pecora AL, Stiff P, Jennis A, et al. Prompt and durable engraftment in two older adult patients with high risk chronic myelogenous leukemia (CML) using ex vivo expanded and unmanipulated unrelated umbilical cord blood. Bone Marrow Transplant 2000;25:797-9. [Crossref] [PubMed]
- Qiu L, Meagher R, Welhausen S, et al. Ex vivo expansion of CD34+ umbilical cord blood cells in a defined serum-free medium (QBSF-60) with early effect cytokines. J Hematother Stem Cell Res 1999;8:609-18. [Crossref] [PubMed]
- Casamayor-Genesca A, Pla A, Oliver-Vila I, et al. Clinical-scale expansion of CD34+ cord blood cells amplifies committed progenitors and rapid scid repopulation cells. N Biotechnol 2017;35:19-29. [Crossref] [PubMed]
- Shpall EJ, Quinones R, Giller R, et al. Transplantation of ex vivo expanded cord blood. Biol Blood Marrow Transplant 2002;8:368-76. [Crossref] [PubMed]
- Jaroscak J, Goltry K, Smith A, et al. Augmentation of umbilical cord blood (UCB) transplantation with ex vivo-expanded UCB cells: results of a phase 1 trial using the AastromReplicell System. Blood 2003;101:5061-7. [Crossref] [PubMed]
- Koller MR, Palsson MA, Manchel I, et al. Long-term culture-initiating cell expansion is dependent on frequent medium exchange combined with stromal and other accessory cell effects. Blood 1995;86:1784-93. [PubMed]
- Wasa M, Satani M, Tanano H, et al. Copper deficiency with pancytopenia during total parenteral nutrition. JPEN J Parenter Enteral Nutr 1994;18:190-2. [Crossref] [PubMed]
- Hirase N, Abe Y, Sadamura S, et al. Anemia and neutropenia in a case of copper deficiency: role of copper in normal hematopoiesis. Acta Haematol 1992;87:195-7. [Crossref] [PubMed]
- Zidar BL, Shadduck RK, Zeigler Z, et al. Observations on the anemia and neutropenia of human copper deficiency. Am J Hematol 1977;3:177-85. [Crossref] [PubMed]
- Huang ZL, Failla ML, Reeves PG. Differentiation of human U937 promonocytic cells is impaired by moderate copper deficiency. Exp Biol Med (Maywood) 2001;226:222-8. [PubMed]
- Peled T, Landau E, Mandel J, et al. Linear polyamine copper chelator tetraethylenepentamine augments long-term ex vivo expansion of cord blood-derived CD34+ cells and increases their engraftment potential in NOD/SCID mice. Exp Hematol 2004;32:547-55. [Crossref] [PubMed]
- Peled T, Mandel J, Goudsmid RN, et al. Pre-clinical development of cord blood-derived progenitor cell graft expanded ex vivo with cytokines and the polyamine copper chelator tetraethylenepentamine. Cytotherapy 2004;6:344-55. [Crossref] [PubMed]
- de Lima M, McMannis J, Gee A, et al. Transplantation of ex vivo expanded cord blood cells using the copper chelator tetraethylenepentamine: a phase I/II clinical trial. Bone Marrow Transplant 2008;41:771-8. [Crossref] [PubMed]
- Stiff PJ, Montesinos P, Peled T, et al. StemEx®(Copper Chelation Based) Ex Vivo Expanded Umbilical Cord Blood Stem Cell Transplantation (UCBT) Accelerates Engraftment and Improves 100 Day Survival In Myeloablated Patients Compared To a Registry Cohort Undergoing Double Unit UCBT: Results Of a Multicenter Study Of 101 Patients With Hematologic Malignancies. Blood 2013;122:295.
- Varnum-Finney B, Xu L, Brashem-Stein C, et al. Pluripotent, cytokine-dependent, hematopoietic stem cells are immortalized by constitutive Notch1 signaling. Nat Med 2000;6:1278-81. [Crossref] [PubMed]
- Delaney C, Heimfeld S, Brashem-Stein C, et al. Notch-mediated expansion of human cord blood progenitor cells capable of rapid myeloid reconstitution. Nat Med 2010;16:232-6. [Crossref] [PubMed]
- Denu JM. Vitamin B3 and sirtuin function. Trends Biochem Sci 2005;30:479-83. [Crossref] [PubMed]
- Narala SR, Allsopp RC, Wells TB, et al. SIRT1 acts as a nutrient-sensitive growth suppressor and its loss is associated with increased AMPK and telomerase activity. Mol Biol Cell 2008;19:1210-9. [Crossref] [PubMed]
- Miura M, Kameda Y. Nicotinamide promotes long-term survival and extensive neurite outgrowth in ultimobranchial C cells cultured from chick embryos. J Comp Neurol 2005;492:334-48. [Crossref] [PubMed]
- Vaca P, Berna G, Martin F, et al. Nicotinamide induces both proliferation and differentiation of embryonic stem cells into insulin-producing cells. Transplant Proc 2003;35:2021-3. [Crossref] [PubMed]
- Bitterman KJ, Anderson RM, Cohen HY, et al. Inhibition of silencing and accelerated aging by nicotinamide, a putative negative regulator of yeast sir2 and human SIRT1. J Biol Chem 2002;277:45099-107. [Crossref] [PubMed]
- Peled T, Shoham H, Aschengrau D, et al. Nicotinamide, a SIRT1 inhibitor, inhibits differentiation and facilitates expansion of hematopoietic progenitor cells with enhanced bone marrow homing and engraftment. Exp Hematol 2012;40:342-55.e1. [Crossref] [PubMed]
- Peled T, Adi S, Peleg I, et al. Nicotinamide Modulates Ex-Vivo Expansion of Cord Blood Derived CD34+ Cells Cultured with Cytokines and Promotes Their Homing and Engraftment in SCID Mice. Blood 2006;108:725.
- Horwitz ME, Chao NJ, Rizzieri DA, et al. Umbilical cord blood expansion with nicotinamide provides long-term multilineage engraftment. J Clin Invest 2014;124:3121-8. [Crossref] [PubMed]
- Wagner JE Jr, Brunstein CG, Boitano AE, et al. Phase I/II Trial of StemRegenin-1 Expanded Umbilical Cord Blood Hematopoietic Stem Cells Supports Testing as a Stand-Alone Graft. Cell Stem Cell 2016;18:144-55. [Crossref] [PubMed]
- Boitano AE, Wang J, Romeo R, et al. Aryl hydrocarbon receptor antagonists promote the expansion of human hematopoietic stem cells. Science 2010;329:1345-8. [Crossref] [PubMed]
- Sauvageau G, Humphries RK. Medicine. The blood stem cell Holy Grail? Science 2010;329:1291-2. [Crossref] [PubMed]
- Deans RJ, Moseley AB. Mesenchymal stem cells: biology and potential clinical uses. Exp Hematol 2000;28:875-84. [Crossref] [PubMed]
- Robinson SN, Simmons PJ, Yang H, et al. Mesenchymal stem cells in ex vivo cord blood expansion. Best Pract Res Clin Haematol 2011;24:83-92. [Crossref] [PubMed]
- Simmons PJ, Torok-Storb B. Identification of stromal cell precursors in human bone marrow by a novel monoclonal antibody, STRO-1. Blood 1991;78:55-62. [PubMed]
- de Lima M, McNiece I, Robinson SN, et al. Cord-blood engraftment with ex vivo mesenchymal-cell coculture. N Engl J Med 2012;367:2305-15. [Crossref] [PubMed]
- Hoggatt J, Singh P, Sampath J, et al. Prostaglandin E2 enhances hematopoietic stem cell homing, survival, and proliferation. Blood 2009;113:5444-55. [Crossref] [PubMed]
- Cutler C, Multani P, Robbins D, et al. Prostaglandin-modulated umbilical cord blood hematopoietic stem cell transplantation. Blood 2013;122:3074-81. [Crossref] [PubMed]
- Xia L, McDaniel JM, Yago T, et al. Surface fucosylation of human cord blood cells augments binding to P-selectin and E-selectin and enhances engraftment in bone marrow. Blood 2004;104:3091-6. [Crossref] [PubMed]
- Robinson SN, Simmons PJ, Thomas MW, et al. Ex vivo fucosylation improves human cord blood engraftment in NOD-SCID IL-2Rgamma(null) mice. Exp Hematol 2012;40:445-56. [Crossref] [PubMed]
- Popat U, Mehta RS, Rezvani K, et al. Enforced fucosylation of cord blood hematopoietic cells accelerates neutrophil and platelet engraftment after transplantation. Blood 2015;125:2885-92. [Crossref] [PubMed]
- Christopherson KW 2nd, Hangoc G, Broxmeyer HE. Cell surface peptidase CD26/dipeptidylpeptidase IV regulates CXCL12/stromal cell-derived factor-1 alpha-mediated chemotaxis of human cord blood CD34+ progenitor cells. J Immunol 2002;169:7000-8. [Crossref] [PubMed]
- Christopherson KW 2nd, Hangoc G, Mantel CR, et al. Modulation of hematopoietic stem cell homing and engraftment by CD26. Science 2004;305:1000-3. [Crossref] [PubMed]
- Velez de Mendizabal N, Strother RM, Farag SS, et al. Modelling the sitagliptin effect on dipeptidyl peptidase-4 activity in adults with haematological malignancies after umbilical cord blood haematopoietic cell transplantation. Clin Pharmacokinet 2014;53:247-59. [Crossref] [PubMed]
- Farag SS, Srivastava S, Messina-Graham S, et al. In vivo DPP-4 inhibition to enhance engraftment of single-unit cord blood transplants in adults with hematological malignancies. Stem Cells Dev 2013;22:1007-15. [Crossref] [PubMed]
- Okas M, Gertow J, Uzunel M, et al. Clinical expansion of cord blood-derived T cells for use as donor lymphocyte infusion after cord blood transplantation. J Immunother 2010;33:96-105. [Crossref] [PubMed]
- Berglund S, Gertow J, Magalhaes I, et al. Cord blood T cells cultured with IL-7 in addition to IL-2 exhibit a higher degree of polyfunctionality and superior proliferation potential. J Immunother 2013;36:432-41. [Crossref] [PubMed]
- Berglund S, Gertow J, Uhlin M, et al. Expanded umbilical cord blood T cells used as donor lymphocyte infusions after umbilical cord blood transplantation. Cytotherapy 2014;16:1528-36. [Crossref] [PubMed]
- Hexner EO, Luger SM, Reshef R, et al. Infusion of CD3/CD28 costimulated umbilical cord blood T cells at the time of single umbilical cord blood transplantation may enhance engraftment. Am J Hematol 2016;91:453-60. [Crossref] [PubMed]
- Doubrovina E, Oflaz-Sozmen B, Prockop SE, et al. Adoptive immunotherapy with unselected or EBV-specific T cells for biopsy-proven EBV+ lymphomas after allogeneic hematopoietic cell transplantation. Blood 2012;119:2644-56. [Crossref] [PubMed]
- Hanley PJ, Cruz CR, Savoldo B, et al. Functionally active virus-specific T cells that target CMV, adenovirus, and EBV can be expanded from naive T-cell populations in cord blood and will target a range of viral epitopes. Blood 2009;114:1958-67. [Crossref] [PubMed]
- Peggs KS, Verfuerth S, Pizzey A, et al. Adoptive cellular therapy for early cytomegalovirus infection after allogeneic stem-cell transplantation with virus-specific T-cell lines. Lancet 2003;362:1375-7. [Crossref] [PubMed]
- Gerdemann U, Keirnan JM, Katari UL, et al. Rapidly generated multivirus-specific cytotoxic T lymphocytes for the prophylaxis and treatment of viral infections. Mol Ther 2012;20:1622-32. [Crossref] [PubMed]
- Papadopoulou A, Gerdemann U, Katari UL, et al. Activity of broad-spectrum T cells as treatment for AdV, EBV, CMV, BKV, and HHV6 infections after HSCT. Sci Transl Med 2014;6:242ra83. [Crossref] [PubMed]
- Naik S, Nicholas SK, Martinez CA, et al. Adoptive immunotherapy for primary immunodeficiency disorders with virus-specific T lymphocytes. J Allergy Clin Immunol 2016;137:1498-505.e1. [Crossref] [PubMed]
- Sun Q, Burton RL, Pollok KE, et al. CD4(+) Epstein-Barr virus-specific cytotoxic T-lymphocytes from human umbilical cord blood. Cell Immunol 1999;195:81-8. [Crossref] [PubMed]
- Park KD, Marti L, Kurtzberg J, et al. In vitro priming and expansion of cytomegalovirus-specific Th1 and Tc1 T cells from naive cord blood lymphocytes. Blood 2006;108:1770-3. [Crossref] [PubMed]
- Hanley PJ, Bollard CM, Brunstein CG. Adoptive immunotherapy with the use of regulatory T cells and virus-specific T cells derived from cord blood. Cytotherapy 2015;17:749-55. [Crossref] [PubMed]
- Hanley PJ, Melenhorst JJ, Nikiforow S, et al. CMV-specific T cells generated from naive T cells recognize atypical epitopes and may be protective in vivo. Sci Transl Med 2015;7:285ra63. [Crossref] [PubMed]
- Milano F, Gooley T, Wood B, et al. Cord-Blood Transplantation in Patients with Minimal Residual Disease. N Engl J Med 2016;375:944-53. [Crossref] [PubMed]
- Dave H, Luo M, Patel S, et al. Rapid Production of Multi-Virus Specific T Cells Targeting BKV, Adenovirus, CMV and EBV for Recipients of Umbilical Cord Blood Transplant. Biol Blood Marrow Transplant 2017;23:S18-391. [Crossref]
- Orentas RJ, Roskopf SJ, Nolan GP, et al. Retroviral transduction of a T cell receptor specific for an Epstein-Barr virus-encoded peptide. Clin Immunol 2001;98:220-8. [Crossref] [PubMed]
- Schub A, Schuster IG, Hammerschmidt W, et al. CMV-specific TCR-transgenic T cells for immunotherapy. J Immunol 2009;183:6819-30. [Crossref] [PubMed]
- Bollard CM, Heslop HE. T cells for viral infections after allogeneic hematopoietic stem cell transplant. Blood 2016;127:3331-40. [Crossref] [PubMed]
- Fernandez I, Ooi TP, Roy K. Generation of functional, antigen-specific CD8+ human T cells from cord blood stem cells using exogenous Notch and tetramer-TCR signaling. Stem Cells 2014;32:93-104. [Crossref] [PubMed]
- Jessen H, Allen TM, Streeck H. How a single patient influenced HIV research--15-year follow-up. N Engl J Med 2014;370:682-3. [Crossref] [PubMed]
- Patel S, Lam S, Cruz CR, et al. Functionally Active HIV-Specific T Cells that Target Gag and Nef Can Be Expanded from Virus-Naive Donors and Target a Range of Viral Epitopes: Implications for a Cure Strategy after Allogeneic Hematopoietic Stem Cell Transplantation. Biol Blood Marrow Transplant 2016;22:536-41. [Crossref] [PubMed]
- Patel S, Lam S, Cruz CR, et al. HIV-Specific T CELLS Expanded from HIV+ and HIV-Naive Donors Target a Range of Viral Epitopes: Implications for a Cure Strategy after Allogeneic HSCT. Biol Blood Marrow Transplant 2017;23:S194-5. [Crossref]
- Merindol N, Grenier AJ, Caty M, et al. Umbilical cord blood T cells respond against the Melan-A/MART-1 tumor antigen and exhibit reduced alloreactivity as compared with adult blood-derived T cells. J Immunol 2010;185:856-66. [Crossref] [PubMed]
- St John LS, Wan L, He H, et al. PR1-specific cytotoxic T lymphocytes are relatively frequent in umbilical cord blood and can be effectively expanded to target myeloid leukemia. Cytotherapy 2016;18:995-1001. [Crossref] [PubMed]
- Weber G, Gerdemann U, Caruana I, et al. Generation of multi-leukemia antigen-specific T cells to enhance the graft-versus-leukemia effect after allogeneic stem cell transplant. Leukemia 2013;27:1538-47. [Crossref] [PubMed]
- Garfall AL, Maus MV, Hwang WT, et al. Chimeric Antigen Receptor T Cells against CD19 for Multiple Myeloma. N Engl J Med 2015;373:1040-7. [Crossref] [PubMed]
- Porter DL, Hwang WT, Frey NV, et al. Chimeric antigen receptor T cells persist and induce sustained remissions in relapsed refractory chronic lymphocytic leukemia. Sci Transl Med 2015;7:303ra139. [Crossref] [PubMed]
- Maude SL, Frey N, Shaw PA, et al. Chimeric antigen receptor T cells for sustained remissions in leukemia. N Engl J Med 2014;371:1507-17. [Crossref] [PubMed]
- Davila ML, Riviere I, Wang X, et al. Efficacy and toxicity management of 19-28z CAR T cell therapy in B cell acute lymphoblastic leukemia. Sci Transl Med 2014;6:224ra25. [Crossref] [PubMed]
- Kochenderfer JN, Dudley ME, Kassim SH, et al. Chemotherapy-refractory diffuse large B-cell lymphoma and indolent B-cell malignancies can be effectively treated with autologous T cells expressing an anti-CD19 chimeric antigen receptor. J Clin Oncol 2015;33:540-9. [Crossref] [PubMed]
- Micklethwaite KP, Savoldo B, Hanley PJ, et al. Derivation of human T lymphocytes from cord blood and peripheral blood with antiviral and antileukemic specificity from a single culture as protection against infection and relapse after stem cell transplantation. Blood 2010;115:2695-703. [Crossref] [PubMed]
- Ma Q, Garber HR, Lu S, et al. A novel TCR-like CAR with specificity for PR1/HLA-A2 effectively targets myeloid leukemia in vitro when expressed in human adult peripheral blood and cord blood T cells. Cytotherapy 2016;18:985-94. [Crossref] [PubMed]
- Huls MH, Figliola MJ, Dawson MJ, et al. Clinical application of Sleeping Beauty and artificial antigen presenting cells to genetically modify T cells from peripheral and umbilical cord blood. J Vis Exp 2013.e50070. [PubMed]
- Jena B, Dotti G, Cooper LJ. Redirecting T-cell specificity by introducing a tumor-specific chimeric antigen receptor. Blood 2010;116:1035-44. [Crossref] [PubMed]
- Pegram HJ, Purdon TJ, van Leeuwen DG, et al. IL-12-secreting CD19-targeted cord blood-derived T cells for the immunotherapy of B-cell acute lymphoblastic leukemia. Leukemia 2015;29:415-22. [Crossref] [PubMed]
- Cohen JL, Trenado A, Vasey D, et al. CD4(+)CD25(+) immunoregulatory T Cells: new therapeutics for graft-versus-host disease. J Exp Med 2002;196:401-6. [Crossref] [PubMed]
- Anderson BE, McNiff JM, Matte C, et al. Recipient CD4+ T cells that survive irradiation regulate chronic graft-versus-host disease. Blood 2004;104:1565-73. [Crossref] [PubMed]
- Taylor PA, Lees CJ, Blazar BR. The infusion of ex vivo activated and expanded CD4(+)CD25(+) immune regulatory cells inhibits graft-versus-host disease lethality. Blood 2002;99:3493-9. [Crossref] [PubMed]
- Zhao D, Zhang C, Yi T, et al. In vivo-activated CD103+CD4+ regulatory T cells ameliorate ongoing chronic graft-versus-host disease. Blood 2008;112:2129-38. [Crossref] [PubMed]
- Tolar J, Hippen KL, Blazar BR. Immune regulatory cells in umbilical cord blood: T regulatory cells and mesenchymal stromal cells. Br J Haematol 2009;147:200-6. [Crossref] [PubMed]
- Godfrey WR, Spoden DJ, Ge YG, et al. Cord blood CD4(+)CD25(+)-derived T regulatory cell lines express FoxP3 protein and manifest potent suppressor function. Blood 2005;105:750-8. [Crossref] [PubMed]
- Brunstein CG, Blazar BR, Miller JS, et al. Adoptive transfer of umbilical cord blood-derived regulatory T cells and early viral reactivation. Biol Blood Marrow Transplant 2013;19:1271-3. [Crossref] [PubMed]
- Brunstein CG, Miller JS, Cao Q, et al. Infusion of ex vivo expanded T regulatory cells in adults transplanted with umbilical cord blood: safety profile and detection kinetics. Blood 2011;117:1061-70. [Crossref] [PubMed]
- Brunstein CG, Miller JS, McKenna DH, et al. Umbilical cord blood-derived T regulatory cells to prevent GVHD: kinetics, toxicity profile, and clinical effect. Blood 2016;127:1044-51. [Crossref] [PubMed]
- Meij P, Jedema I, Zandvliet ML, et al. Effective treatment of refractory CMV reactivation after allogeneic stem cell transplantation with in vitro-generated CMV pp65-specific CD8+ T-cell lines. J Immunother 2012;35:621-8. [Crossref] [PubMed]
- Cobbold M, Khan N, Pourgheysari B, et al. Adoptive transfer of cytomegalovirus-specific CTL to stem cell transplant patients after selection by HLA-peptide tetramers. J Exp Med 2005;202:379-86. [Crossref] [PubMed]
- Feucht J, Opherk K, Lang P, et al. Adoptive T-cell therapy with hexon-specific Th1 cells as a treatment of refractory adenovirus infection after HSCT. Blood 2015;125:1986-94. [Crossref] [PubMed]
- McKenna DH Jr, Sumstad D, Kadidlo DM, et al. Optimization of cGMP purification and expansion of umbilical cord blood-derived T-regulatory cells in support of first-in-human clinical trials. Cytotherapy 2017;19:250-62. [Crossref] [PubMed]
Cite this article as: Mehta RS, Dave H, Bollard CM, Shpall EJ. Engineering cord blood to improve engraftment after cord blood transplant. Stem Cell Investig 2017;4:41.


