Beyond THC: the new generation of cannabinoid designer drugs
- 1 Institute of Neuroscience – Cagliari National Research Council of Italy, @ Department of Neuroscience, Cittadella Universitaria di Monserrato, Cagliari, Italy
- 2 Centre of Excellence “Neurobiology of Dependence,” Cittadella Universitaria di Monserrato, University of Cagliari, Monserrato, Italy
- 3 Department of Neuroscience, Cittadella Universitaria di Monserrato, University of Cagliari, Monserrato, Italy
Synthetic cannabinoids are functionally similar to delta9-tetrahydrocannabinol (THC), the psychoactive principle of cannabis, and bind to the same cannabinoid receptors in the brain and peripheral organs. From 2008, synthetic cannabinoids were detected in herbal smoking mixtures sold on websites and in “head shops” under the brand name of Spice Gold, Yucatan Fire, Aroma, and others. Although these products (also known as “Spice drugs” or “legal highs”) do not contain tobacco or cannabis, when smoked they produce effects similar to THC. Intoxication, withdrawal, psychosis, and death have been recently reported after consumption, posing difficult social, political, and health challenges. More than 140 different Spice products have been identified to date. The ability to induce strong cannabis-like psychoactive effects, along with the fact that they are readily available on the Internet, still legal in many countries, marketed as natural safe substances, and undetectable by conventional drug screening tests, has rendered these drugs very popular and particularly appealing to young and drug-naïve individuals seeking new experiences. An escalating number of compounds with cannabinoid receptor activity are currently being found as ingredients of Spice, of which almost nothing is known in terms of pharmacology, toxicology, and safety. Since legislation started to control the synthetic cannabinoids identified in these herbal mixtures, many new analogs have appeared on the market. New cannabimimetic compounds are likely to be synthesized in the near future to replace banned synthetic cannabinoids, leading to a “dog chasing its tail” situation. Spice smokers are exposed to drugs that are extremely variable in composition and potency, and are at risk of serious, if not lethal, outcomes. Social and health professionals should maintain a high degree of alertness for Spice use and its possible psychiatric effects in vulnerable people.
Introduction
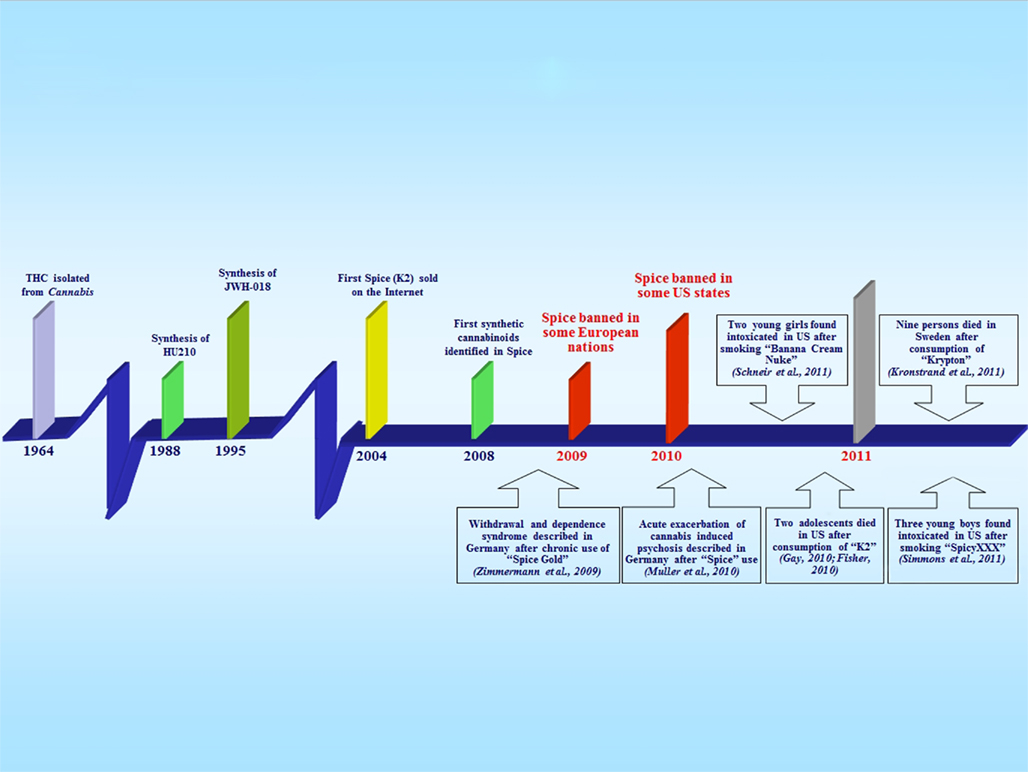
Cannabis is one of the oldest drugs of abuse and its consumption is still high worldwide. During the past two decades, knowledge of its pharmacology and the role of the endocannabinoid system in brain function and physiology has improved greatly (Thakur et al., 2009; Alger and Kim, 2011). Despite its long history of use and abuse for both medical and recreational purposes, a new generation of synthetic cannabinoids has recently emerged on the market, which are sold on the Internet as herbal mixtures under the brand names of “Spice,” “Spice Gold,” “Spice Diamond,” “Arctic Spice,” “Silver,” “Aroma,” “K2,” “Genie,” “Scene” or “Dream,” and advertised as incense products, meditation potpourris, bath additives, or air fresheners. These products are often referred to as “herbal highs” or “legal highs” because of their legal status and purported natural herbal make-up. They are distributed in the form of dried leaves or resin, although more recently powdery products have also begun to emerge (Kikura-Hanajiri et al., 2011), and are sold without age restriction in metal-foil sachets, usually containing 3 g of vegetable matter, to which one or more of the synthetic cannabinoids have been added. Spice is typically smoked, using a pipe or by rolling in a cigaret paper, but can also be ingested as an infusion, or inhaled. These novel and increasingly popular recreational drugs first appeared on websites and in specialized shops (“head shops,” which sell paraphernalia for cannabis users) around 2004, if not earlier (Dresen et al., 2010), and are sold as mild hallucinogens with prominent cannabis-like effects. They soon became popular in Central European countries, began to catch the attention of a broader public in 2007, and gained a high degree of popularity in 2008. Consequently, they have also attracted the attention of the European Monitoring Centre for Drugs and Drug Addiction (EMCDDA) early warning system on new drugs (Early Warning System, 2009; EMCDDA, 2009). However, it was not before the end of 2008 that two synthetic cannabinoids were identified for the first-time as the main active (not declared) ingredients of an herbal blend called “Spice”: the C8 homolog of the non-classical cannabinoid CP-47,497, CP-47,497-C8, and a cannabimimetic aminoalkylindole called JWH-018 (Auwärter et al., 2009; Uchiyama et al., 2009a). At the beginning of 2009, legislation in several European countries (Austria, Germany, France, Luxembourg, Poland, Lithuania, Sweden, and Estonia) subjected all products containing these substances to the Narcotics Law, so that Spice and the other cannabinoid-containing “natural” mixtures were no longer accessible in head shops and online stores (Figure 1). In the same year, following advice from the Advisory Council on the Misuse of Drugs (ACMD, 2009) and the EMCDDA, the Misuse of Drugs Act 1971 was amended and classified synthetic cannabinoids as controlled substances in the UK (UK Statutory Instrument, 2009). Far from stopping their sale, prohibition rather had the effect of facilitating the generation of new follow-up designer cannabimimetic substances: the aminoalkylindole JWH-073 (Auwärter et al., 2009; Lindigkeit et al., 2009), the hexyl homolog JWH-019 (Dresen et al., 2011), and the two more recent aminoalkylindoles, JWH-250 (Westphal et al., 2010) and JWH-398 (Hudson et al., 2010). During 2009, the potent synthetic cannabinoid agonist HU-210 was identified in Spice products in the UK (EMCDDA, 2009). Starting from the middle of 2010, the EMCDDA warned about the presence of JWH-015 in an herbal mixture called “Topaz” that is sold in Austria, along with the discovery of a methyl derivative of JWH-073 (1-butyl-3-(1-(4-methyl)naphthoyl)indole), JWH-122 (1-pentyl-3-(4-methyl-1-naphthoyl)indole) being a methyl derivative of JWH-018, and AM-694 (1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole) as the first halogenated aminoalkylindole found in an herbal mixture (Ernst et al., 2011). On March 2011, the Drug Enforcement Administration (DEA) issued the final order to temporarily ban five synthetic cannabinoids, namely JWH-018, JWH-073, JWH-200, CP-47,497, and CP-47,497-C8. Currently, there are no more doubts that these Spice-like products are no longer limited to European Countries but rather have spread worldwide, from Ukraine, to Taiwan, to the USA (Vardakou et al., 2010), and a growing number of countries are currently implementing their own law or policy of controlling at one or more of these synthetic cannabinoids (Drug Policy Alliance, 2011). In Europe, the emergence of Spice drugs has became a major alarm for the Europol’s Organised Crime Threat Assessment (OCTA, 2011), while in the USA starting from February 2011, the Uniform Code of Military Justice (UCMJ) banned Spice and US Air Force is now screening urine tests for synthetic cannabinoids (Air Force Times, 2011; UCMJ, 2011).
Drug Queen of the Web
The presence of websites dedicated to the use of recreational controlled drugs as well as the use of cyberspace for the assessment of the drug abuse market are not recent phenomena (Rawaf and Schifano, 2000; Schifano et al., 2003, 2006). Yet, the role played by the Internet as one of the major markets for novel designer drugs is increasingly alarming (Corazza et al., 2011). To date, new Spice products that purportedly contain synthetic cannabinoids appear in the online market on a regular basis and their popularity has grown rapidly in the past few years (Fabrizio Schifano, personal communication). Tracking the world wide web, it is clear that the vast majority of legal highs are purchased online, which partly explains why Spice use is so widespread and causes social and medical concern (Burley, 2008; Vardakou et al., 2011). There are an increasing number of websites where users can order Spice blends or pure JWH compounds without age restriction or any type of control. Amazingly, with a few clicks of a mouse, many highly psychoactive substances can be obtained cheaply and legally (Schmidt et al., 2011). On a growing number of blogs, users report their own mixtures and testing procedures, describe subjective side effects, and express their preference for their favorite smoking blends. That is, in a smoking blend competition, “Spice Diamond” was elected as the best smoking product among 41 different mixtures (Vardakou et al., 2010). Looking at the different Internet fora, it appears that users are perfectly conscious of the cannabis-like effects of these herbal blends; they know that their psychoactive properties are mainly due to synthetic cannabinoids, and more surprisingly, in most cases, they are aware that these compounds have never been tested for human consumption.
A dramatic online snapshot of the Spice phenomenon as an emerging trend has been recently given by an important web mapping program, the Psychonaut Web Mapping Project, a European Commission-funded project involving researchers from seven European countries (Belgium, Finland, Germany, Italy, Norway, Spain, and UK), which aims to develop a web scanning system to identify newly marketed psychoactive compounds, and their combinations (e.g., ketamine and Spice, cannabis and Spice), on the basis of the information available on the Internet (Psychonaut Web Mapping Research Group, 2010). As a major result of the Project, a new and updated web-based database is now widely accessible to implement a regular monitoring of the web for novel and recreational drugs1.
By monitoring fora, blogs, and chats, as well as e-newsgroups, chat rooms, mailing lists, e-newsletters, and bulletin boards in eight languages (Dutch, English, Finnish, German, Italian, Norwegian, Spanish, and Swedish), Professor Fabrizio Schifano (the Scientific Coordinator of the Psychonaut Web Mapping Project) and collaborators have found that online users mostly appreciate Spice products for their psychoactive effects, lack of detection in body fluids, ease of online access, and legal status, although some consumers have reported unpleasant side effects, such as paranoia, cramps, strong headache, mild hallucinations, and vomiting (Schifano et al., 2009). Although the provenance of these herbal mixture is often not clear, the project has identified potential wholesalers and manufacturers of Spice, including a UK company (Psyche Deli) and a Dutch company (Zonged.eu/MultiNETional), although some Spice products seem to be imported from China (Jack, 2009). Notably, not only Spice mixtures but also related merchandise, such as an informative CD explaining their psychoactive effects, can be found on sale on eBay (Aurazendoctor, 2009). It is clear that the Internet offers an overabundance of drug-related data that are constantly one step ahead of those available to clinicians and law enforcement authorities (Boyer et al., 2001). Likewise, the poor quality of product information provided to consumers is particularly worrying, because the majority of the websites have inconsistent information available to users (Hillebrand et al., 2010).
Why Cannabinoid Designer Drugs are so Popular
They Induce Psychoactive Effects
According to discussions on retailers’ websites, Spice smokers find drug effects similar to those of marijuana, leading to the hypothesis that many users smoke it as a legal alternative to cannabis. Apparently labeled as incense, the herbal constituents listed on the packaging of Spice have been deliberately contaminated with synthetic cannabinoids (mainly JWH-018) to induce cannabimimetic effects (Steup, 2009). Thus, while the natural ingredients of these herbal mixtures seem not to possess psychoactive properties per se, synthetic cannabinoids are probably mixed into a solvent and then sprayed on a plant-derived base for delivery, leading to a final product with potent cannabis-like properties (Vardakou et al., 2010). Their agonistic activity on the CB1 receptor is responsible for elevating mood and inducing a feeling of well-being. Some Spice users have reported effects similar to or even stronger than those obtained by smoking cannabis, such as physical relaxation, changes in perception, and mild euphoria. The higher potency of action of these synthetic cannabinoids might be explained by in vitro experiments that have suggested that, while THC acts as a partial agonist on the CB1 receptor, JWH-018 acts as a full and potent agonist (Atwood et al., 2010). Moreover, compared with THC, JWH-018 possesses approximately a fourfold higher affinity to the cannabinoid CB1 receptor and 10-fold higher affinity to CB2 receptor (Aung et al., 2000; Huffman and Padgett, 2005). With respect to other products containing non-controlled plants and fungi and marketed as legal highs (e.g., Salvia divinorum or khat), Spice blends better satisfy users’ expectations, in that their psychoactive effects are perceived to be even stronger than cannabis (Griffiths et al., 2010). Regrettably, safety information provided is sparse and of uncertain utility, with only a few products warnings about potential adverse effects or drug interactions.
They are Legal
Spices are often referred to as legal highs, in that they are neither controlled by the 1971 Misuse of Drugs Act, nor licensed for legal use such as alcohol and nicotine. Both the use and possession of these drugs have been long officially authorized, and their supply tolerated as long as they are sold for purposes other than human consumption. The relatively recent identification of the first synthetic cannabinoids as not declared ingredients of Spice marked a significant turning point in this situation. Indeed, although structurally distinct from THC, synthetic cannabinoids are part of the well-characterized aminoalkylindole class of ligands that also bind and activate CB1 receptors. Although their government regulation is still inconsistent or even lacking in many countries, Spice products are currently controlled in 14 European nations (Austria, Denmark, Estonia, France, Germany, Ireland, Italy, Latvia, Lithuania, Luxembourg, Poland, Romania, Sweden, and UK), where they are classified as pharmaceuticals or narcotics. Yet, they are still legal and uncontrolled in the remaining parts of Europe and many other countries, leading to heavy global marketing.
In the USA, some states (Alabama, Arkansas, Georgia, Kansas, Kentucky, and Missouri) have taken legislative action against the distribution and use of Spice, and until recently, only one synthetic cannabinoid, namely HU-210, was considered a Schedule I substance (unsafe, highly abused, no medical usage). On November 24, 2010, the United States Drug Enforcement Administration temporarily added to the list the following synthetic cannabinoids: JWH-018, JWH-073, JWH-200, CP-47,497, and cannabicyclohexanol (US Department of Justice Drug Enforcement Administration Drugs and Chemicals of Concern, 2010). JWH compounds (i.e., JWH-018, JWH-015, and JWH-073) are also currently unregulated in New Zealand and are easily obtainable in head shops and from many websites (Every-Palmer, 2011).
However, regulatory mechanisms are weak and difficult to enforce when controlled products are available on the Internet, because online retailers can evade easily national jurisdiction and supply Spice products to other countries but not their own. In addition, experience has shown that, as legal authorities adopt control measures, other synthetic cannabinoids are soon added to existing Spice-like products, suggesting that the producers expect prohibition and are ready to synthesize an assortment of substitutes (Lindigkeit et al., 2009). It looks like the producers are moving onto the next product, always one step ahead of the law (Dargan et al., 2011). This is not totally unexpected because there is a high demand for legal highs (United Nations Office on Drugs and Crime, 2011; Zawilska, 2011), implying that it will be satisfied by products containing chemical ingredients that are not yet prohibited. Lack of consistency in the measures adopted to control the market, ranging from medical legislation to formal drug control instruments, is another major point of concern in monitoring and responding to worldwide circulation of Spice (ACMD, 2009; McLachlan, 2009).
They are Ready Available and Highly Attractive
Marketed under the generic brand name of Spice, an increasing number of herbal mixtures are sold mainly on the Internet, and in some countries, in dedicated shops that offer legal alternatives to prohibited drugs. The price is affordable also by young people, roughly €9–12 per gram: each sachet typically contains 3 g of smoking mixture, sufficient for around eight joints, and costs €27–36. As reported in a warning editorial by Griffiths et al. (2010), the Spice phenomenon represents a clear example of how globalization brings major challenges to the control of new drugs marketing, in that a user living in a country where Spice is controlled can buy it from a foreign retailer.
It is noteworthy that the multicolored packaging of these herbal blends is very attractive and highly sophisticated; many of them have a wide-open-eye imprint and circulate under exotic brand names, such as “Tropical Synergy” or “Yucatan Fire” (Sobolevsky et al., 2010). Although the product label often states that the product is “not for human consumption” or “for aromatherapy only,” the composite blend of ingredients listed on the pack suggests the opposite, suggesting that the purpose of such a statement is to elude the interest of the medicines and healthcare products regulatory agencies. Because of their packaging resembling incense or tea and their scented smell, Spice products are far less noticeable as drugs, are not easily identified by parents or carers, and can comfortably be consumed at home. Thus, they are extremely tempting for young people that are willing to try cannabis but are afraid of the legal consequences and/or the reputation.
They are Perceived as Safe Drugs
Commercial advertisements describing Spice as “natural herbs” or “harmless incense blend” are very colorful and use intuitive (figurative) language, resulting in greater attractiveness for vulnerable individuals who might not otherwise smoke cannabis, in particular adolescents wishing to have a “safe” experience. Lack of safety information could lead to the incorrect supposition that herbal mixtures are safe, especially amongst first-time users. These herbal blends typically have a pleasurable smell and taste (i.e., honey or vanilla), are delivered in attractive packaging, often as pre-rolled cigarets, and are marketed as “incense cones.” For some people, undesirable psychoactive effects and public perception of cannabinoid use could represent major limitations in the use of marijuana: Spice circumvents these limits by appearing as a safer alternative to cannabis. People that have been warned against using cannabis for legislative (i.e., after a period in prison or a forensic hospital) or medical (i.e., predisposition to psychotic illness) reasons might also find Spice a safe (and undetectable) drug to use. Moreover, Spice products represent a tempting alternative for those who have experienced adverse effects from smoking marijuana (ACMD, 2009; EMCDDA, 2009). However, all Spice products introduced into the market lack any published in vivo testing, even in animal models, and very little information is available in international medical databases.
They are not Easily Detectable in Urine and Blood Samples
Most of the synthetic cannabinoids added as not-listed ingredients to Spice products are very difficult to detect by commonly used drug screening procedures. Apart from the analogs of THC such as HU-210, the structure of these new synthetic cannabinoids differs from that of THC, so that they probably will not trigger a positive test for cannabinoids in immunoassays of body fluids. This has important consequences, because it encourages not only cannabis users but also curious people with no previous experience of illicit drugs to use these products to attain cannabis-like effects, without having to fear prosecution. Furthermore, wherever drug screening is routinely performed to guarantee abstinence from drugs (i.e., hospital or institutions carrying out detoxification, forensic psychiatric centers), people can be motivated to substitute cannabis with Spice products. Maximal research effort is currently focusing on the development of new analytical procedure for measuring urinary concentrations of synthetic cannabinoids and several potential metabolites of each (Beuck et al., 2011; Grigoryev et al., 2011; Hudson and Ramsey, 2011; Moran et al., 2011).
What Do They Contain?
It is not easy to determine exactly what is in Spice products, due to the lack of reference samples and the presence of masking agents of natural origin such as tocopherol (vitamin E), eugenol, or fatty acids (Dresen et al., 2010), which are commonly added to confuse identification. Spice is supposed to contain up to 15 different vegetal compounds, which gives rise to a wide variety of drug combinations (Zuba et al., 2011), among which are the psychoactive herbs known as “Wild Dagga” (Leonotis leonurus) and “Indian warrior” (Pedicularis densiflora). Potentially psychoactive alkaloids, such as betonicine, aporphine, leonurine nuciferine, or nicotine, are often declared as ingredients in these products; yet, only some herbal mixtures have the constituents stated, and most samples of Spice contain inert vegetable matter. The synthetic psychoactive compounds added to Spice were initially almost unknown substances, which forensic laboratories had difficulties in recognizing and finding reference samples. A decisive improvement in the identification of these chemicals is the recent development of a GC–MS screening procedure that combines high chromatographic resolution with the existence of well-established libraries, offering huge collections of spectra that can be adapted by adding spectra of emerging psychoactive compounds that have probably been added to herbal mixtures (Auwärter et al., 2009; Dresen et al., 2010, 2011).
The active compounds present in Spice products are placed principally at the surface of the herbal ingredients, and the extraction procedure is able to remove them without significant contamination by the vegetable components of the herb. Unfortunately, trafficking and detection of these drugs is hampered by the fact that the exact content of many Spice products still remains unpredictable, because it changes constantly over time as a reaction to prevention and legal actions, leading to an ever-expanding array of synthetic cannabinoids being available on the market. It is clear that comprehension of the clinical pharmacology of these compounds is essential for practitioners and scientists to discriminate the relative toxicity associated with the different synthetic cannabinoid mixtures and routes of administration. The major psychoactive ingredients of Spice products are illustrated in Figure 2.
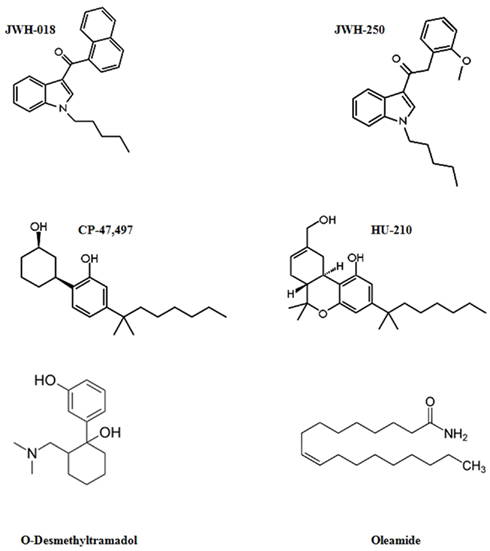
Figure 2. Structures of most common psychoactive ingredients of Spice products: synthetic cannabinoids (JWH-018, JWH-250, CP-47,497, HU-210), μ-opioid agonists (O-Desmethyltramadol), and fatty acid derivatives with cannabinoid-like activity (Oleamide).
Cannabinoids
Unlike cannabis, Spice products do not contain the phytocannabinoids cannabidiol (rarely THC) but synthetic cannabinoid drugs, which originate from four chemically distinct groups: (i) the JWH compounds, synthesized by John W. Huffman (JWH) in the 1980s, of which JWH-018 is the most studied and best characterized to date; (ii) the CP-compounds, a cyclohexylphenol series synthesized by Pfizer in the 1970s, with the identified CP-47,497 and its modified version CP-47,497-C8 (obtained by extending the dimethylheptyl side chain to dimethyloctyl); (iii) the HU-compounds, synthesized in the 1960s at the Hebrew University; and (iv) the benzoylindoles, such as AM-694 and RCS-4 (Huffman et al., 2008; EMCDDA, 2009; Lindigkeit et al., 2009; Uchiyama et al., 2009b, 2010, 2011a,b; United States Drug Enforcement Administration, 2009; Hudson et al., 2010; Nakajima et al., 2011). The show binding affinities for the CB1 and/or the CB2 receptors (see EMCDDA, 2009 for a comprehensive review), are lipid soluble and non-polar, and consist of 22 to 26 carbon atoms, which explains why they volatilize readily when smoked. Contrary to nabilone, a synthetic analog of tetrahydrocannabinol approved by the US Food and Drug Administration (FDA) to treat chemotherapy-induced nausea and vomiting, no therapeutic effects have been documented so far for synthetic cannabinoids detected in these herbal mixtures.
The family of the JWH compounds is the most numerous and, although their chemical structures differ greatly from those of THC, they have a higher affinity to CB1 and/or CB2 receptors and are more potent than THC (Huffman et al., 2003; Huffman, 2009). Conversely, JWH-015 [(2-methyl-1-propyl-1H-indol-3-yl)-1-naphthalenylmethanone] acts as a selective CB2 receptor agonist (Aung et al., 2000). At the end of 2008, the synthetic cannabinoid naphthoylindole, JWH-018, and the cyclohexylphenol CP-47,497, along with one of its active homologs, CP-47,497-C8, were detected using nuclear magnetic resonance spectroscopy (Auwärter et al., 2009). JWH-018 (naphthalen-1-yl-(1-pentylindol-3-yl)methanone) was first synthesized during an analysis aiming at developing new cannabimimetic indole compounds with potential therapeutic effects comparable with those of THC (Chin et al., 1999). It belongs to the aminoalkylindole family and has been shown to have a binding affinity for the CB1 receptors in the low nanomolar range (∼9 nM; Aung et al., 2000; Atwood et al., 2010). In cannabinoid receptor expressing CHO cells, JWH-018 inhibits forskolin-stimulated cAMP production (Chin et al., 1999), whereas in HEK293 cells stably expressing this receptor, it was recently found to activate multiple cannabinoid receptor signaling pathways, including the phosphorylation of ERK1/2 mitogen activated protein kinase and the internalization of CB1 receptors (Atwood et al., 2010). Specifically, JWH-018 dose-dependently inhibits glutamate release in autaptic excitatory hippocampal neurons, probably acting on the CB1 receptor, an effect reversed by administration of the CB1 receptor antagonist rimonabant (Atwood et al., 2010). In vivo studies showing that JWH-018 induces analgesia, catalepsy, hypomotility, and hypothermia, namely the tetrad of behaviors classically caused by cannabinoids administration (Wiley et al., 1998), have confirmed that this compound acts as a potent and effective CB1 receptor agonist. Specifically, JWH-018 displayed fourfold affinity to the CB1 receptor and about 10-fold affinity to the CB2 receptor compared with THC (Aung et al., 2000; Huffman et al., 2005). It is worth nothing that unlike metabolites of most synthetic cannabinoids, JWH-018 hydroxylated metabolites retain in vitro and in vivo activity at CB1 receptors (Brents et al., 2011), a finding that in conjunction with the higher CB1 receptor affinity and activity relative to THC may contribute to the greater prevalence of adverse effects observed with JWH-018-containing products relative to marijuana. Other JWH compounds have been identified in herbal mixtures, such as JWH-250, which shows high affinity for the CB1 and CB2 receptors (Dresen et al., 2010, 2011), and the butyl homolog of JWH-018, JWH-073 (naphthalen-1-yl-(1-butylindol-3-yl)methanone), which seems to bind more specifically to the CB1 receptor (Wiley et al., 1998; Aung et al., 2000). The latter has been recently shown to act similarly to JWH-018, although it is less potent in inhibiting neurotransmission and slower in producing internalization of cannabinoid receptors (Atwood et al., 2011). Several studies have reported a recent decrease in the content of JWH-018 in Spice products, replaced by JWH-073 (Lindigkeit et al., 2009) or other synthetic psychoactive cannabinoids (Hudson et al., 2010; Uchiyama et al., 2010, 2011a), of which JWH-398 is the most recently identified in the UK and Germany (Vardakou et al., 2010). JWH-398 was found to be a very potent non-selective CB1/CB2 receptor agonist (Huffman, 2009), while in a recent study, the N-alkyl-3-(1-naphthoyl)indole JWH-122, a very potent CB1 receptor agonist with a structure closely related to JWH-018 and JWH-073, has been identified as a new ingredient of commercial samples of a Spice product called “Lava Red” (Ernst et al., 2011).
The CP-47,497 cannabinoid compound (2-(1R,3S)-3-hydroxycyclohexyl]-5-(2-methyloctan-2-yl)phenol) lacks the classical cannabinoid chemical structure (tricyclic benzopyran system) and is 3–28 times more potent than THC (Weissman et al., 1982). Like its homolog cannabicyclohexanol (CP-47,497-C8), it shows higher affinity to the receptor CB1 compared to CB2 (Auwärter et al., 2009), and has THC-like activity in animals (Weissman et al., 1982; Compton et al., 1992). The concentration–response curve of CP-47,497-C8 in inhibiting neurotransmission in autaptic hippocampal neuron cultures is nearly identical to that described for JWH-018 (Atwood et al., 2010, 2011).
The classical cannabinoid HU-210 [(6aR,10aR)-9-(hydroxymethyl)-6,6-dimethyl-3-(2-methyloctan-2-yl)-6a,7,10,10a-tetra-hydrobenzo[c] chromen-1-ol)], whose agonistic activity on the CB1 receptor has been long recognized, is an ingredient of herbal mixtures in the UK and USA European Monitoring Centre for Drugs and Drug Addiction (EMCDDA, 2009), where it has been placed under control since 2009 and 2010, respectively. This synthetic analog of THC was shown to be a full non-selective agonist at the CB1 and CB2 receptors, and to possess intrinsic affinities for cannabinoid receptors that exceed those of the high-efficacy agonists, CP 55,940 and WIN 55,212-2 (Howlett et al., 2002). Notably, the pharmacological effects of HU-210 in vivo are also exceptionally long lasting, and in animal models it has been shown to negatively affect learning (Ferrari et al., 1999) and memory (Robinson et al., 2007; Mackowiak et al., 2009) processes as well as sexual behavior (Ferrari et al., 2000).
Among the benzoylindoles, AM-694 [(1-(5-fluoropentyl)-3-(2-iodobenzoyl)indole)] binds strongly to CB1 and CB2 receptors, and now represents an example of the latest synthetic cannabinoid agonists added to Spice that is currently available on the UK market, but still not controlled by current UK legislation (Dargan et al., 2011). Another hazardous benzoylindole is RCS-4 [(4-methoxyphenyl)(1-pentyl-1H-indol-3-yl)methanone)], which is a synthetic JWH-018 cannabinoid analog with uncertain biological activity that, under the name of “Devil Smoke,” is currently used in combination with JWH-073 (Drugs-Forum, 2011). On March 11, 2011, it was banned as euphoriant substance by the Danish Ministry of Health (2011).
It seems reasonable to hypothesize that additional compounds beside the above-mentioned might also contribute to the behavioral and subjective effects produced by smoking Spice, and that their different pharmacology might explain the different psychoactive effects experienced after smoking Spice. Although the marijuana-like effects of smoked Spice products are probably due to activation of CB1 receptor, the potential role of CB2 receptors in such effects is still to be investigated. Regrettably, cannabinoids identified so far in Spice products are believed to be only the tip of an iceberg; the first of a larger number of synthesized substances with cannabis-like effects mediated by their agonist activity at the CB1 (and/or CB2) receptor. Currently, more than 100 compounds with cannabimimetic activities are waiting for identification.
Opioids
Besides cannabinoids, other psychoactive substances can be part of Spice products, such as the synthetic opioid O-desmethyltramadol (Dresen et al., 2010). This opioid is an active metabolite of the opioid tramadol, a centrally acting analgesic drug with suspected abuse liability (Raffa, 2008). Very recently, O-desmethyltramadol was found as an ingredient of a Spice-like blend called “Krypton,” in combination with Kratom (Mitragyna speciosa), an Asiatic medicinal plant that has been used as an herbal drug for a long time (Arndt et al., 2011; Philipp et al., 2011). Mitragynine, an alkaloid present in Kratom, acts as a μ-opioid receptor agonist, and when combined with O-desmethyltramadol, another potent μ-opioid agonist, can lead to fatal consequences. Indeed, in less than 1 year, consumption of Krypton had fatal results and caused the unintentional deaths of nine persons (Kronstrand et al., 2011).
Other Substances
Oleamide (cis-9,10-octadecenoamide), a fatty acid derivative with cannabinoid-like activity (Leggett et al., 2004) and hypnotic properties (Fedorova et al., 2001) is one of the most frequent non-cannabinoid ingredients associated with Spice products (Dresen et al., 2010). In association with JWH-018, oleamide is present in an herbal mixture sold as “Aroma” (Every-Palmer, 2011). In particular, it was found that “Aroma” contained the highest concentration of oleamide and the second highest concentration of JWH-018 (Uchiyama et al., 2010). Harmine and harmaline, two reversible inhibitors of the monoamine oxidase enzyme with stimulating central effects (Fortunato et al., 2009, 2010), have also been found in one of these products in combination with myristicin and asarone (Dresen et al., 2010, 2011). Benzophenone (HM 40) is another undeclared substance found in an herbal mixture, although most likely it was not added purposely but rather should be considered a contamination from synthesis (Dresen et al., 2010, 2011).
Many other ingredients are listed on the Spice packets, with their combinations greatly varying in number and concentration, often depending on the country of distribution. For example, in a packet of Spice called “Banana Cream Nuke” bought in an USA smoke shop, the following ingredients were listed: alfalfa, blue violet, nettle leaf, comfrey leaf, Gymnema sylvestre, passion flower leaf, horehound, and neem leaf (Schneir et al., 2011). Notably, this product caused acute intoxication in two young girls, probably due to the co-presence of THC, JWH-018, and JWH-073 identified among 15 other synthetic cannabinoids, whereas none of the listed ingredients were detectable (Schneir et al., 2011), with the only exception of passion flower (Passiflora sp.) that is well known to possess anxiolytic properties (Dhawan et al., 2004). Conversely, some packets of Spice sold in the UK declare beach bean (Canavalia maritima or Canavalia rosea), blue lotus (Nelumbo nucifera), and dwarf skullcap (Scutellaria nana) as ingredients of the mixture, for which no safety data are available (Burley, 2008). Moreover, in Germany, in the past 2 years, head shops were selling different varieties of herbal mixtures by combining the above-mentioned plants with white or blue water lily (Nymphaea alba or Nymphaea caerulea), Indian Warrior (Pedicularis densiflora), Lion’s Ear (also known as Lion’s Tail or Wild Dagga; L. leonurus), Maconha Brava (Zornia latifolia), and Honeyweed or Siberian Motherwort (Leonurus sibiricus; Teske et al., 2010). Other plants commonly used in Spice products in combination with synthetic cannabinoids included Marshmallow (Althaea officinalis) and Dog Rose or Rosehip (Rosa canina; Seely et al., 2011). Not surprisingly, no natural cannabinoids were declared as constituents.
What are Their Main Effects?
In a growing number of Internet blogs, Spice is described by users as able to exert strong cannabis-like effects, but inter- and intra-batch variations, both in terms of substances present and their quantity, have also resulted in accidental overdosing that requires hospitalization (Auwärter et al., 2009). Worryingly, very little is known about its pharmacology and toxicology in humans, and virtually nothing has been investigated thus far about the health implications of its use, either in humans or animals, which hampers appropriate medical treatment of Spice-induced side effects. The carcinogenic potential caused by inhaling smoke containing these substances has also not been evaluated (EMCDDA, 2009). Only limited data on the pharmacological properties of CP-47,497 in animal models and on the metabolism of JWH-015 in rat liver microsomes are available (Compton et al., 1992; Zhang et al., 2006). It is noteworthy that CP-47,497 generalized with THC in drug discrimination studies in rats, that is, it produces subjective effects similar to those of THC, with an absolute threshold dose 3–14 times lower than that of THC (Weissman et al., 1982). Very recently, JWH-018 and CP-47,497 were found to significantly decrease the locomotor activity and increase the electroencephalogram power spectra in rats (Uchiyama et al., 2011b).
In general, the desired effects of Spice include a sense of empathy and well-being. However, there is an increasing number of clinical reports describing patients presenting for emergency medical care after smoking Spice products, the most common symptoms being nausea, anxiety, agitation/panic attacks, tachycardia, paranoid ideation, and hallucinations (Piggee, 2009; Banerji et al., 2010; Bebarta et al., 2010; Vearrier and Osterhoudt, 2010). On the Internet, it is possible to find a quantity of self-reports of users experiencing anxiety and psychotic symptoms after using synthetic cannabinoids2. Finally, in the literature, there is one published case report of tolerance and withdrawal phenomena (Zimmermann et al., 2009), another of drug-induced psychosis (Müller et al., 2010), and two clinical studies conducted on psychotic patients (Every-Palmer, 2010, 2011).
Central Effects and Cognitive Deficits
Spice blends are often described as energizing, euphoric, and disinhibiting (Schifano et al., 2009), which are likely among the most desirable effects pursued by users. However, halting speech and avoidant eye contact were observed in a young student who smoked Spice for 3 weeks (Benford and Caplan, 2011). Moreover, after chronic (8 months) daily use, Spice can induce serious cognitive impairment (Zimmermann et al., 2009). Loss of consciousness and confusion have also been described, as well as unresponsiveness, seizures, agitation, and irritation (Seely et al., 2011; Simmons et al., 2011b).
Emotional Alterations
Anxiety is one of the main side effects experienced during acute intoxication, which resolves within 1–2 h after consumption (Schneir et al., 2011). A sense of extreme anxiety and sudden depression has been reported during withdrawal from chronic Spice use (Zimmermann et al., 2009). Paranoia and hallucinations have been observed in some patients (Banerji et al., 2010; Bebarta et al., 2010; Simmons et al., 2011a). Alterations in mood and perception after Spice have also been described (Auwärter et al., 2009), and two studies has associated the use of synthetic cannabinoids with exacerbation of cannabis-induced psychosis (Müller et al., 2010; Benford and Caplan, 2011). Interestingly, unlike cannabis, Spice blends do not contain cannabidiol, a phytocannabinoids known to possess anxiolytic properties, which is able to reduced anxiety in both animals (Guimarães et al., 1990; Moreira et al., 2006) and humans (Bergamaschi et al., 2011; Crippa et al., 2011). More importantly, cannabidiol displays high potency as an antagonist of CB1 and CB2 receptor agonists (Thomas et al., 2007; Pertwee, 2008), and is able to revert not only THC-induced social withdrawal in rats (Malone et al., 2009) but also THC-induced anxiety in normal volunteers (Zuardi et al., 1982), suggesting that lack of this cannabinoid in Spice drugs may exacerbate the detrimental effects of these herbal mixtures on emotion and sociability.
Dependence and Withdrawal
To the best of our knowledge, only one case report in Germany has described thus far a withdrawal syndrome after discontinuation from smoking Spice (Zimmermann et al., 2009). Specifically, withdrawal phenomena and a dependence syndrome have been described after chronic consumption of an herbal mixture called “Spice Gold” (typically containing CP-47,497-C8 and JWH-018) in a 20-year-old man, who had a history of smoking this product daily for 8 months as the only relief from his internal unrest and nervousness. He found Spice relaxing and sedative, with psychoactive effects very similar to those of cannabis. He entered hospital voluntarily, requesting medical treatment for detoxification of Spice after experiencing a similar syndrome a few weeks earlier during a phase of abstinence owing to a short supply. Internal unrest and profuse sweating were among the first symptoms observed by doctors, followed by drug craving, nocturnal nightmares, tremor, and headache. Other physical withdrawal symptoms included palpitation, nausea and vomiting, and were not dissimilar from those described during cannabis withdrawal (Budney and Hughes, 2006). Besides a clear withdrawal syndrome, a diagnosis of dependency was confirmed by the development of drug tolerance (the patient had to increase rapidly the dose from 1 to 3 g/day), persistence of drug craving (he felt a continuous strong desire for the drug), the continuous urge to consume it despite the adverse consequences (cognitive impairments and risk of losing his professional training position), and the scarce attention to other interests or duties (participation in practical work).
Psychotic Effects
The link between cannabis use and the occurrence of psychotic episodes is widely recognized, although it has not been determined yet whether abuse of the drug in psychotic patients antedates the onset of the pathology or it is a consequence of the disorder (Arseneault et al., 2002). Indeed, regular cannabis use is thought to increase the risk of developing psychosis and to facilitate the manifestation of the disorder in vulnerable individuals. On the other hand, patients smoke cannabis to self-medicate the negative symptoms of schizophrenia or the side effects of antipsychotic medications. The great medical interest in examining the effects of these synthetic cannabinoids on the psychotic population is therefore not surprising. Few data are available on the psychological and other risks of synthetic cannabinoids; nevertheless, despite the limited number of clinical observations, in the Internet fora, a growing number of users have reported experiencing psychotic symptoms after smoking Spice.
A first case report described the effects of Spice on a 25-year-old man who had a history of cannabis-induced recurrent psychotic episodes (Müller et al., 2010). It was found that Spice triggered not only acute exacerbation of cannabis-induced recurrent psychotic episodes, but also the manifestation of new symptoms, such as recurrent paranoid hallucinations (Müller et al., 2010). To such an acute reactivation of symptoms after abuse of Spice could have contributed the absence of cannabidiol, which is presumed to have antipsychotic potency (Zuardi et al., 2006; Zullino et al., 2007), thus suggesting a higher potency for psychosis of these substances. In line with this, relapses following the use of a Spice product in psychotic patients have been reported by forensic services (Every-Palmer, 2010). More recently, psychotic relapse after smoking Spice was confirmed in 15 psychotic New Zealand patients, all familiar with a locally available JWH-018-containing product called “Aroma” (Every-Palmer, 2011). Intriguingly, no one of these patients reported withdrawal symptoms or physical distress after using the Spice product, three of them described developing some tolerance to the product, and 13 acknowledged having smoked “Aroma” as a cannabis substitute. A latest study described the case of a young student experiencing severe anxiety, paranoia, and both visual and auditory hallucinations after repeated (3 weeks) use of Spice (Benford and Caplan, 2011). Thus, evidence seems to indicate that Spice products can precipitate psychosis in vulnerable individuals, implying the necessity of advising people with risk factors for psychosis against using synthetic cannabinoids.
Peripheral Effects
Although gastrointestinal effects, such as nausea, vomiting, and retching, are the most common after consumption of Spice, cardiovascular effects, such as extremely elevated heart rate and blood pressure, chest pain, and cardiac ischemia are among the most dangerous consequences (Canning et al., 2010; Schneir et al., 2011; Seely et al., 2011). Occasional inappropriate laughter, injected conjunctiva, xerostomia, and nystagmus have been described as well (Auwärter et al., 2009; Schneir et al., 2011). Spice also induces metabolic effects, such as hypokalemia, hyperglycemia, and acidosis, and autonomic effects, such as fever and mydriasis (Seely et al., 2011; Simmons et al., 2011a).
Lethal Effects
Contrary to the partial action of THC at the CB1 receptor, synthetic cannabinoids identified so far in Spice products have been shown to act as full agonists with increased potency, thus leading to longer durations of action and an increased likelihood of adverse effects. Although limited at the moment, some life-threatening symptoms have been reported by subjects that use these products as marijuana substitutes, and coma and suicide attempts have been reported after smoking the dangerous spice drug K2 (Missouri Department of Health and Senior Services, 2010). Dramatically, two adolescents died in the USA after ingestion of a Spice product called “K2,” one due to a coronary ischemic event (Fisher, 2010), and the other committed suicide due to the unbearable sense of extreme anxiety (Gay, 2010). The non-cannabinoid ingredient of many Spice products, namely the opioid agonist O-desmethyltramadol, when used in combination with Kratom (as in the mixture known as Krypton), may have lethal consequences (Kronstrand et al., 2011).
Concluding Remarks
Spice drugs include a large range of products sold as “ethno drugs” or legal substitutes for cannabis since 2004. From the end of 2008, some potent synthetic cannabinoids, such as JWH-018 and CP-47,497, were identified as psychoactive ingredients of these herbal blends, and some countries placed them under control. Due to their powerful psychoactivity, ready availability on the Internet, legal status, and non-detection in drug testing, Spice products have acquired an unexpected popularity, especially among youngest and first-time consumers, including college students (Hu et al., 2011). Synthetic cannabinoids currently available on the market have been shown to induce severe peripheral and central effects, including drug dependence and psychosis. Yet, these products provide very limited safety information about their effects and possible health consequences, so that uninformed users risk serious adverse effects. This renders it very complicated, if not impossible, for health professionals and clinicians to carry out accurate assessments of possible drug-related medical and psychiatric consequences of their use. The emergence of the Internet as the major player in shaping the international Spice market has led to significant public health concerns. Continuous monitoring of herbal mixtures available online is essential for timely detection of new chemicals, which will continue to be developed as a reaction to the newly implemented control measures (Uchiyama et al., 2009b, 2010, 2011a; Dresen et al., 2010, 2011; Dowling and Regan, 2011; Westphal et al., 2011).
Future Perspectives
Very limited information is available on the safety of the Spice ingredients in humans, and the occurrence of serious health damage in their abusers is highly probable. Rapid detection and identification of new synthetic cannabinoids in human urine and blood samples would greatly restrict their use and diffusion. Up to the beginning of 2010, the first methodologies for the quantification of synthetic cannabinoids in human serum have been developed and validated in accordance with conventional screening protocols based on enzymatic hydrolysis, liquid–liquid extraction, and liquid chromatography/electrospray tandem mass spectrometry analysis (Müller et al., 2010; Teske et al., 2010). Researchers should continue to develop rapid and reliable detection screening procedures, because they would be crucial for medical staff in assisting patients at the emergency units, to psychiatrists in recognizing and treating psychiatric symptoms, and to police authorities in assessing fitness to drive. Evidence that the synthetic cannabinoids are not detectable in human body fluids underlines the need to elucidate their metabolic pathways and identify their metabolites, which could shed light on their pharmacokinetics and toxicity (Wintermeyer et al., 2010). Cannabinoids have been classified as doping substances by the World Anti-Doping Agency (WADA, 2011), thus, screening for the synthetic cannabinoids and their major metabolites could also be applied to human urine doping controls (Müller et al., 2010).
In countries where synthetic cannabinoids are under control, incessant monitoring of the manufacture, distribution, and use of marketed Spice-like products is necessary. Given the worldwide spread of these herbal mixtures, an international cooperation system is mandatory for sharing analytical information and improving monitoring of the global drug market. In countries where synthetic cannabinoids are marketed legally, accurate labeling of products containing psychoactive compounds should be requested, so that users can be conscious of the potential risks associated. In both cases, however, it will be an ongoing challenge to detect synthetic cannabinoids in herbal mixtures and to include them in analytical methods. Future research should focus on the study of their pharmacological effects, both at the central (dependence, psychosis, anxiety, depression) and peripheral (tremor, nausea, tachycardia, headache) levels, as well as on the evaluation of the health consequences of smoking Spice repeatedly. This is also to avoid the risk of banning compounds with not health hazarding profile which would probably be replaced quickly by more dangerous substances (Hammersley, 2010). More importantly, understanding of the neurobiological bases of such compounds activity might encourage the development of synthetic cannabinoids that produce therapeutic effects with a minimum of psychoactive effects, such as the synthetic THC dronabinol (Marinol), the synthetic THC analog nabilone (Cesamet), and the standardized cannabis extract (Sativex).
Conflict of Interest Statement
The authors declare that the research was conducted in the absence of any commercial or financial relationships that could be construed as a potential conflict of interest.
Footnotes
References
Advisory Council on the Misuse of Drugs (ACMD). (2009). Consideration of the Major Cannabinoid Agonists. London: Home Office.
Air Force Times. (2011). AF Using Urine Tests to Detect “Spice” Use. Available at: http://www.airforcetimes.com/news/2011/03/air-force-using-urine- tests-for-spice-031211w/ [accessed August 9, 2011].
Alger, B. E., and Kim, J. (2011). Supply and demand for endocannabinoids. Trends Neurosci. 3, 304–315.
Arndt, T., Claussen, U., Güssregen, B., Schröfel, S., Stürzer, B., Werle, A., and Wolf, G. (2011). Kratom alkaloids and O-desmethyltramadol in urine of a “Krypton” herbal mixture consumer. Forensic Sci. Int. 208, 47–52.
Arseneault, L., Cannon, M., Poulton, R., Murray, R., Caspi, A., and Moffitt, T. E. (2002). Cannabis use in adolescence and risk for adult psychosis: longitudinal prospective study. BMJ 325, 1212–1213.
Atwood, B. K., Huffman, J., Straiker, A., and Mackie, K. (2010). JWH018, a common constituent of “spice” herbal blends, is a potent and efficacious cannabinoid CB1 receptor agonist. Br. J. Pharmacol. 160, 133–140.
Atwood, B. K., Lee, D., Straiker, A., Widlanski, T. S., and Mackie, K. (2011). CP47,497-C8 and JWH073, commonly found in “spice” herbal blends, are potent and efficacious CB(1) cannabinoid receptor agonists. Eur. J. Pharmacol. 659, 139–145.
Aung, M. M., Griffin, G., Huffman, J. W., Wu, M., Keel, C., Yang, B., Showalter, V. M., Abood, M. E., and Martin, B. R. (2000). Influence of the N-1 alkyl chain length of cannabimimetic indoles upon CB1 and CB2 receptor binding. Drug Alcohol Depend. 60, 133–140.
Aurazendoctor. (2009). Make Your Own Herbal Spice Gold Smoke Blends eBook CD, eBay, ID Number: 270362517411. Available at: http://cgi.ebay.com/Make-Your-Own -Herbal-Spice-Gold-Smoke-Blends- eBook-CD_W0QQitemZ270362517 411 [accessed March 24, 2009].
Auwärter, V., Dresen, S., Weinmann, W., Müller, M., Pütz, M., and Ferreirós, N. (2009). “Spice” and other herbal blends: harmless incense or cannabinoid designer drugs? J. Mass Spectrom. 44, 832–837.
Banerji, S., Deutsch, C. M., and Bronstein, A. C. (2010). Spice ain’t so nice. Clin. Toxicol. (Phila.) 48, 632.
Bebarta, V. S., Varney, S., and Sessions, D. (2010). Spice: a new “legal” herbal mixture abused by young active duty military personnel. Clin. Toxicol. (Phila.) 48, 632.
Benford, D. M., and Caplan, J. P. (2011). Psychiatric sequelae of spice, k2, and synthetic cannabinoid receptor agonists. Psychosomatics 52, 295.
Bergamaschi, M. M., Queiroz, R. H., Chagas, M. H., de Oliveira, D. C., De Martinis, B. S., Kapczinski, F., Quevedo, J., Roesler, R., Schröder, N., Nardi, A. E., Martín-Santos, R., Hallak, J. E., Zuardi, A. W., and Crippa, J. A. (2011). Cannabidiol reduces the anxiety induced by simulated public speaking in treatment-naïve social phobia patients. Neuropsychopharmacology 36, 1219–1226.
Beuck, S., Möller, I., Thomas, A., Klose, A., Schlörer, N., Schänzer, W., and Thevis, M. (2011). Structure characterisation of urinary metabolites of the cannabimimetic JWH-018 using chemically synthesised reference material for the support of LC-MS/MS-based drug testing. Anal. Bioanal. Chem. 401, 493–505.
Boyer, E. W., Shannon, M., and Hibberd, P. L. (2001). Web sites with misinformation about illicit drugs. N. Engl. J. Med. 345, 469–471.
Brents, L. K., Reichard, E. E., Zimmerman, S. M., Moran, J. H., Fantegrossi, W. E., and Prather, P. L. (2011). Phase I hydroxylated metabolites of the K2 synthetic cannabinoid JWH-018 retain in vitro and in vivo cannabinoid 1 receptor affinity and activity. PLoS One 6, e21917. doi: 10.1371/journal.pone.0021917
Budney, A. J., and Hughes, J. R. (2006). The cannabis withdrawal syndrome. Curr. Opin. Psychiatry 19, 233–238.
Burley, B. (2008). Doctors Worries Over Legal Highs. BBC Newsbeat Oct 7, 2008. Available at: http://news.bbc. co.uk/newsbeat/hi/health/newsid_7656000/7656172.stm [accessed May 3, 2011].
Canning, J. C., Ruha, A.-M., Pierce, R., Torrey, M., and Reinhart, S. J. (2010). Severe GI distress after smoking JWH-018. Clin. Toxicol. (Phila.) 48, 618.
Chin, C. N., Murphy, J. W., Huffman, J. W., and Kendall, D. A. (1999). The third transmembrane helix of the cannabinoid receptor plays a role in the selectivity of aminoalkylindoles for CB2, peripheral cannabinoid receptor. J. Pharmacol. Exp. Ther. 291, 837–844.
Compton, D. R., Johnson, M. R., Melvin, L. S., and Martin, B. R. (1992). Pharmacological profile of a series of bicyclic cannabinoid analogs: classification as cannabimimetic agents. J. Pharmacol. Exp. Ther. 260, 201–209.
Corazza, O., Schifano, F., Farre, M., Deluca, P., Davey, Z., Torrens, M., Demetrovics, Z., Di Furia, L., Flesland, L., Siemann, H., Skutle, A., Van Der Kreeft, P., and Scherbaum, N. (2011). Designer drugs on the internet: a phenomenon out-of-control? The emergence of hallucinogenic drug Bromo-Dragonfly. Curr. Clin. Pharmacol. 6, 125–129.
Crippa, J. A., Derenusson, G. N., Ferrari, T. B., Wichert-Ana, L., Duran, F. L., Martin-Santos, R., Simões, M. V., Bhattacharyya, S., Fusar-Poli, P., Atakan, Z., Santos Filho, A., Freitas-Ferrari, M. C., McGuire, P. K., Zuardi, A. W., Busatto, G. F., and Hallak, J. E. (2011). Neural basis of anxiolytic effects of cannabidiol (CBD) in generalized social anxiety disorder: a preliminary report. J. Psychopharmacol. (Oxford) 25, 121–130.
Danish Ministry of Health. (2011). Amendment of executive order on euphoriant substances 11 March 2011. Available at: http://laegemiddelstyrelsen.dk/en/service-menu/product-areas/euphoriant-substances/nyheder-om-euforiserende-stoffer/amendment-of-executive-order-on-euphoria-march 2011 [accessed August 8, 2011].
Dargan, P. I., Hudson, S., Ramsey, J., and Wood, D. M. (2011). The impact of changes in UK classification of the synthetic cannabinoid receptor agonists in “spice.” Int. J. Drug Policy 22, 274–277.
Dhawan, K., Dhawan, S., and Sharma, A. (2004). Passiflora: a review update. J. Ethnopharmacol. 94, 1–23.
Dowling, G., and Regan, L. (2011). A method for CP 47, 497 a synthetic non-traditional cannabinoid in human urine using liquid chromatography tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 253–259.
Dresen, S., Ferreirós, N., Pütz, M., Westphal, F., Zimmermann, R., and Auwärter, V. (2010). Monitoring of herbal mixtures potentially containing synthetic cannabinoids as psychoactive compounds. J. Mass Spectrom. 45, 1095–1232.
Dresen, S., Kneisel, S., Weinmann, W., Zimmermann, R., and Auwärter, V. (2011). Development and validation of a liquid chromatography-tandem mass spectrometry method for the quantitation of synthetic cannabinoids of the aminoalkylindole type and methanandamide in serum and its application to forensic samples. J. Mass Spectrom. 46, 163–171.
Drug Enforcement Administration. (2011). Schedules of Controlled Substances: Temporary Placement of Five Synthetic Cannabinoids Into Schedule I. Federal Register 2011, 76(40). Available at: http://www.deadiversion.usdoj.gov/fed_regs/rules/2011/fr0301.htm [accessed August 8, 2011].
Drug Policy Alliance. (2011). Legislation to Ban K2/Spice, “Bath Salts” and Other Synthetic Drugs Sailing Through Congress Today. Available at: http://www.drugpolicy.org/news/2011/07/legislation-ban-k2spice-bath-salts-and-other-synthetic-drugs-sailing-through-congress-t [accessed August 9, 2011].
Drugs-Forum. (2011). Herbal incense recipe (JWH-073 + RCS-4). Available at: http://www.drugs-forum.com/forum/showthread.php?t=146867 [accessed June 3, 2011].
Early Warning System. (2009). Available at: http://www.emcdda.europa.eu/themes/new-drugs/early-warning [accessed October 23, 2009].
Ernst, L., Schiebel, H. M., Theuring, C., Lindigkeit, R., and Beuerle, T. (2011). Identification and characterization of JWH-122 used as new ingredient in “spice-like” herbal incenses. Forensic Sci. Int. 208, 31–35.
European Monitoring Centre for Drugs and Drug Addiction (EMCDDA). (2009). Understanding the “Spice” Phenomenon. Lisbon: EMCDDA; 2009. Available at: http://www.emcdda.europa.eu/html.cfm/index90917EN.html [accessed April 23, 2011].
Every-Palmer, S. (2010). Warning: legal synthetic cannabinoid-receptor agonists such as JWH018 may precipitate psychosis in vulnerable individuals. Addiction 105, 1859–1860.
Every-Palmer, S. (2011). Synthetic cannabinoid JWH-018 and psychosis: an explorative study. Drug Alcohol Depend. 117, 152–157.
Fedorova, I., Hashimoto, A., Fecik, R. A., Hedrick, M. P., Hanus, L. O., Boger, D. L., Rice, K. C., and Basile, A. S. (2001). Behavioral evidence for the interaction of oleamide with multiple neurotransmitter systems. J. Pharmacol. Exp. Ther. 299, 332–342.
Ferrari, F., Ottani, A., and Giuliani, D. (2000).Inhibitory effects of the cannabinoid agonist HU 210 on rat sexual behaviour. Physiol. Behav. 69, 547–554.
Ferrari, F., Ottani, A., Vivoli, R., and Giuliani, D. (1999). Learning impairment produced in rats by the cannabinoid agonist HU 210 in a water-maze task. Pharmacol. Biochem. Behav. 64, 555–561.
Fisher, W.G. (2010). Inhaled Incense “K2” May Cause Heart Damage. Available at: http://drwes.blogspot. com/2010/08/inhaled-incense-k2- may-cause-heart.html? [accessed June 2, 2011].
Fortunato, J. J., Réus, G. Z., Kirsch, T. R., Stringari, R. B., Fries, G. R., Kapczinski, F., Hallak, J. E., Zuardi, A. W., Crippa, J. A., and Quevedo, J. (2010). Effects of beta-carboline harmine on behavioral and physiological parameters observed in the chronic mild stress model: further evidence of antidepressant properties. Brain Res. Bull. 81, 491–496.
Fortunato, J. J., Réus, G. Z., Kirsch, T. R., Stringari, R. B., Stertz, L., Kapczinski, F., Pinto, J. P., Hallak, J. E., Zuardi, A. W., Crippa, J. A., and Quevedo, J. (2009). Acute harmine administration induces antidepressive-like effects and increases BDNF levels in the rat hippocampus. Prog. Neuropsychopharmacol. Biol. Psychiatry 33, 1425–1430.
Gay, M. (2010). Synthetic Marijuana Spurs State Bans. In The New York Times, July 10. Available at: http://www.nytimes.com/2010/07/11/us/11k2.html [accessed April 3, 2011].
Griffiths, P., Sedefov, R., Gallegos, A., and Lopez, D. (2010). How globalization and market innovation challenge how we think and respond to drug use: “spice” a case study. Addiction 105, 951–953.
Grigoryev, A., Savchuk, S., Melnik, A., Moskaleva, N., Dzhurko, J., Ershov, M., Nosyrev, A., Vedenin, A., Izotov, B., Zabirova, I., and Rozhanets, V. (2011). Chromatography-mass spectrometry studies on the metabolism of synthetic cannabinoids JWH-018 and JWH-073, psychoactive components of smoking mixtures. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 879, 1126–1136.
Guimarães, F. S., Chiaretti, T. M., Graeff, F. G., and Zuardi, A. W. (1990). Antianxiety effect of cannabidiol in the elevated plus-maze. Psychopharmacology (Berl.) 100, 558–559.
Hammersley, R. (2010). Dangers of banning spice and the synthetic cannabinoid agonists. Addiction 105, 373.
Hillebrand, J., Olszewski, D., and Sedefov, R. (2010). Legal highs on the Internet. Subst. Use Misuse 45, 330–340.
Howlett, A. C., Barth, F., Bonner, T. I., Cabral, G., Casellas, P., Devane, W. A., Felder, C. C., Herkenham, M., Mackie, K., Martin, B. R., Mechoulam, R., and Pertwee, R. G. (2002). International union of pharmacology. XXVII. Classification of cannabinoid receptors. Pharmacol. Rev. 54, 161–202.
Hu, X., Primack, B. A., Barnett, T. E., and Cook, R. L. (2011). College students and use of K2: an emerging drug of abuse in young persons. Subst. Abuse Treat. Prev. Policy 6, 16–19.
Hudson, S., and Ramsey, J. (2011). The emergence and analysis of synthetic cannabinoids. Drug Test Anal. 3, 466–478.
Hudson, S., Ramsey, J., King, L., Timbers, S., Maynard, S., Dargan, P. I., and Wood, D. M. (2010). Use of high-resolution accurate mass spectrometry to detect reported and previously unreported cannabinomimetics in “herbal high” products. J. Anal. Toxicol. 34, 252–260.
Huffman, J. W. (2009). “Cannabimimetic indoles, pyrroles, and indenes: structure-activity relationships and receptor interactions,” in The Cannabinoid Receptors, ed. P. H. Reggio (New York, Humana Press), 49–94.
Huffman, J. W., Mabon, R., Wu, M. J., Lu, J., Hart, R., Hurst, D. P., Reggio, P. H., Wiley, J. L., and Martin, B. R. (2003). 3-Indolyl-1-naphthylmethanes: new cannabimimetic indoles provide evidence for aromatic stacking interactions with the CB1 cannabinoid receptor. Bioorg. Med. Chem. 11, 539–549.
Huffman, J. W., and Padgett, L. W. (2005). Recent developments in the medicinal chemistry of cannabinomimetic indoles, pyrroles and indenes. Curr. Med. Chem. 12, 1395–1411.
Huffman, J. W., Thompson, A. L., Wiley, J. L., and Martin, B. R. (2008). Synthesis and pharmacology of 1-deoxy analogs of CP-47,497 and CP-55,940. Bioorg. Med. Chem. 16, 322–335.
Huffman, J. W., Zengin, G., Wu, M. J., Lu, J., Hynd, G., Bushell, K., Thompson, A. L., Bushell, S., Tartal, C., Hurst, D. P., Reggio, P. H., Selley, D. E., Cassidy, M. P., Wiley, J. L., and Martin, B. R. (2005). Structure–activity relationships for 1-alkyl-3-(1-naphthoyl)indoles at the cannabinoid CB(1) and CB(2) receptors: steric and electronic effects of naphthoyl substituents. New highly selective CB(2) receptor agonists. Bioorg. Med. Chem. 13, 89–93.
Jack, A. (2009). The Story of Spice. The Financial Times Online, February 13. Available at: http://www.ft. com/cms/s/2/1721e2da-f8a0-11dd- aae8-000077b07658.html [accessed May 5, 2011].
Kikura-Hanajiri, R., Uchiyama, N., and Goda, Y. (2011). Survey of current trends in the abuse of psychotropic substances and plants in Japan. Leg. Med. (Tokyo) 13, 109–115.
Kronstrand, R., Roman, M., Thelander, G., and Eriksson, A. (2011). Unintentional fatal intoxications with mitragynine and o-desmethyltramadol from the herbal blend krypton. J. Anal. Toxicol. 35, 242–247.
Leggett, J. D., Aspley, S., Beckett, S. R., D’Antona, A. M., Kendall, D. A., and Kendall, D. A. (2004). Oleamide is a selective endogenous agonist of rat and human CB1 cannabinoid receptors. Br. J. Pharmacol. 141, 253–262.
Lindigkeit, R., Boehme, A., Eiserloh, I., Luebbecke, M., Wiggermann, M., Ernst, L., and Beuerle, T. (2009). Spice: a never ending story? Forensic Sci. Int. 191, 58–63.
Malone, D. T., Jongejan, D., and Taylor, D. A. (2009). Cannabidiol reverses the reduction in social interaction produced by low dose delta(9)-tetrahydrocannabinol in rats. Pharmacol. Biochem. Behav. 93, 91–96.
Mackowiak, M., Chocyk, A., Dudys, D., and Wedzony, K. (2009). Activation of CB1 cannabinoid receptors impairs memory consolidation and hippocampal polysialylated neural cell adhesion molecule expression in contextual fear conditioning. Neuroscience 158, 1708–1716.
Missouri Department of Health and Senior Services. (2010). Health Advisory (3.05.10): K2 Synthetic Marijuana Use among Teenagers and Young Adults in Missouri Missouri. Available at: http://health.mo.gov/emergencies/ert/alertsadvisories/index.php [accessed August 8, 2011].
Moran, C. L., Le, V. H., Chimalakonda, K. C., Smedley, A. L., Lackey, F. D., Owen, S. N., Kennedy, P. D., Endres, G. W., Ciske, F. L., Kramer, J. B., Kornilov, A. M., Bratton, L. D., Dobrowolski, P. J., Wessinger, W. D., Fantegrossi, W. E., Prather, P. L., James, L. P., Radominska-Pandya, A., and Moran, J. H. (2011). Quantitative measurement of JWH-018 and JWH-073 metabolites excreted in human urine. Anal. Chem. 83, 4228–4236.
Moreira, F. A., Aguiar, D. C., and Guimarães, F. S. (2006). Anxiolytic-like effect of cannabidiol in the rat Vogel conflict test. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 1466–1471.
Müller, H., Sperling, W., Köhrmann, M., Huttner, H. B., Kornhuber, J., and Maler, J. M. (2010). The synthetic cannabinoid Spice as a trigger for an acute exacerbation of cannabis induced recurrent psychotic episodes. Schizophr. Res. 118, 309–310.
Nakajima, J., Takahashi, M., Seto, T., and Suzuki, J. (2011). Identification and quantitation of cannabimimetic compound JWH-250 as an adulterant in products obtained via the internet. Forensic Toxicol. 29, 51–55.
Organised Crime Threat Assessment (OCTA). (2011). Europol Public Information. Available at: http://www.europol.europa.eu/content/publication/octa-2011-eu-organised-crime-threat-assessment-655 [accessed August 9, 2011].
Pertwee, R. G. (2008). The diverse CB1 and CB2 receptor pharmacology of three plant cannabinoids: delta9-tetrahydrocannabinol, cannabidiol and delta9-tetrahydrocannabivarin. Br. J. Pharmacol. 153, 199–215.
Philipp, A. A., Meyer, M. R., Wissenbach, D. K., Weber, A. A., Zoerntlein, S. W., Zweipfenning, P. G., and Maurer, H. H. (2011). Monitoring of kratom or Krypton intake in urine using GC-MS in clinical and forensic toxicology. Anal. Bioanal. Chem. 400, 127–135.
Psychonaut Web Mapping Research Group. (2010). Psychonaut Web Mapping Project: Final Report. London: Institute of Psychiatry, King’s College London.
Raffa, R. B. (2008). Basic pharmacology relevant to drug abuse assessment: tramadol as example. J. Clin. Pharmacol. Ther. 33, 101–108.
Rawaf, S., and Schifano, F. (2000). The internet, children, young people and substance misuse: co-ordinated approaches are needed to minimise the use of Ecommerce to obtain drugs and prevent substance misuse. Public Health Med. 2, 96.
Robinson, L., Goonawardena, A. V., Pertwee, R. G., Hampson, R. E., and Riedel, G. (2007). The synthetic cannabinoid HU210 induces spatial memory deficits and suppresses hippocampal firing rate in rats. Br. J. Pharmacol. 151, 688–700.
Schifano, F., Corazza, O., Deluca, P., Davey, Z., Di Furia, L., Flesland, L., Mannonen, M., Pagani, S., Peltoniemi, T., Pezzolesi, C., Scherbaum, N., Siemann, H., Skutle, A., Torrens, M., and Van Der Kreeft, P. (2009). Psychoactive drug or mystical incense? Overview of the online available information on spice products. Int. J. Cult. Ment. Health 2, 137–144.
Schifano, F., Deluca, P., Baldacchino, A., Peltoniemi, T., Scherbaum, N., Torrens, M., Farre, M., Flores, I., Rossi, M., Eastwood, D., Guionnet, C., Rawaf, S., Agosti, L., Di Furia, L., Brigada, R., Majava, A., Siemann, H., Leoni, M., Tomasin, A., Rovetto, F., and Ghodse, A. H. (2006). Drugs on the web; the psychonaut 2002 EU project. Prog. Neuropsychopharmacol. Biol. Psychiatry 30, 640–646.
Schifano, F., Leoni, M., Martinotti, G., Rawaf, S., and Rovetto, F. (2003). Importance of cyberspace for the assessment of the drug abuse market: preliminary results from the psychonaut 2002 project. Cyberpsychol. Behav. 6, 405–410.
Schmidt, M. M., Sharma, A., Schifano, F., and Feinmann, C. (2011). “Legal highs” on the net-evaluation of UK-based Websites, products and product information. Forensic Sci. Int. 206, 92–97.
Schneir, A. B., Cullen, J., and Ly, B. T. (2011). “Spice” girls: synthetic cannabinoid intoxication. J. Emerg. Med. 40, 296–299.
Seely, K. A., Prather, P. L., James, L. P., and Moran, J. H. (2011). Marijuana-based drugs: innovative therapeutics or designer drugs of abuse? Mol. Interv. 11, 36–51.
Simmons, J., Cookman, L., Kang, C., and Skinner, C. (2011a). Three cases of “spice” exposure. Clin. Toxicol. (Phila.) 49, 431–433.
Simmons, J. R., Skinner, C. G., Williams, J., Kang, C. S., Schwartz, M. D., and Wills, B. K. (2011b). Intoxication from smoking “spice.” Ann. Emerg. Med. 57, 187–188.
Sobolevsky, T., Prasolov, I., and Rodchenkov, G. (2010). Detection of JWH-018 metabolites in smoking mixture post-administration urine. Forensic Sci. Int. 200, 141–147.
Steup, C. (2009). Drugs Shipped as Incense Seized as DHL. Wilmington News Journal. Available at: http://usualredant.de/drogen/download/analyse-thc-pharm-Spicejwh-018.pdf [accessed March 4, 2009].
Teske, J., Weller, J.P., Fieguth, A., Rothämel, T., Schulz, Y., and Tröger, H.D. (2010). Sensitive and rapid quantification of the cannabinoid receptor agonist naphthalen-1-yl-(1-pentylindol-3-yl)methanone (JWH-018) in human serum by liquid chromatography–tandem mass spectrometry. J. Chromatogr. B Analyt. Technol. Biomed. Life Sci. 878, 265–269.
Thakur, G. A., Tichkule, R., Bajaj, S., and Makriyannis, A. (2009). Latest advances in cannabinoid receptor agonists. Expert Opin. Ther. Pat. 19, 1647–1673.
Thomas, A., Baillie, G. L., Phillips, A. M., Razdan, R. K., Ross, R. A., and Pertwee, R. G. (2007). Cannabidiol displays unexpectedly high potency as an antagonist of CB1 and CB2 receptor agonists in vitro. Br. J. Pharmacol. 150, 613–623.
Uchiyama, N., Kawamura, M., Kikura-Hanajiri, R., and Goda, Y. (2011a). Identification and quantitative analyses of two cannabimimetic phenylacetylindoles, JWH-251 and JWH-250, and four cannabimimetic naphthoylindoles, JWH-081, JWH-015, JWH-200 and JWH-073, as designer drugs in illegal products. Forensic Toxicol. 29, 25–37.
Uchiyama, N., Kikura-Hanajiri, R., Matsumoto, N., Huang, Z. L., Goda, Y., and Urade, Y. (2011b). Effects of synthetic cannabinoids on electroencephalogram power spectra in rats. Forensic Sci. Int. 1. doi: 10.1016/j.forsciint.2011.05.005. [Epub ahead of print].
Uchiyama, N., Kikura-Hanajiri, R., Kawahara, N., and Goda, Y. (2009a). Identification of a cannabimimetic indole as a designer drug in a herbal product. Forensic Toxicol. 27, 61–66.
Uchiyama, N., Kikura-Hanajiri, R., Kawahara, N., Haishima, Y., and Goda, Y. (2009b). Identification of a cannabinoid analog as a new type of designer drug in a herbal product. Chem. Pharm. Bull. 57, 439–441.
Uchiyama, N., Kikura-Hanajiri, R., Ogata, J., and Goda, Y. (2010). Chemical analysis of synthetic cannabinoids as designer drugs in herbal products. Forensic Sci. Int. 198, 31–38.
UK Statutory Instrument. (2009). No. 3209. Dangerous Drugs. The Misuse of Drugs Act 1971 (Amendment) Order 2009. Available at: http://www.opsi.gov.uk/si/si2009/pdf/uksi 20093209 en.pdf [accessed August 13, 2010].
Uniform Code of Military Justice (UCMJ). (2011). UCMJ Bans Spice. Available at: http://court-martial.com/spice/ [accessed August 9, 2011].
United Nations Office on Drugs and Crime (UNODC). (2011). World Drug Report, 2010. Available at: http://www.idpc. net/publications/unodc-world-drug- report-2011 [accessed August 9, 2011].
United States Drug Enforcement Administration, Office of Forensic Sciences (2009). “Spice”-Plant Material(S) Laced With Synthetic Cannabinoids or Cannabinoid Mimicking Compounds. Microgram Bulletin 42(3). Available at: http://www.justice.gov/dea/programs/forensicsci/microgram/mg0309/mg0309.html [accessed June 1, 2010].
US Department of Justice Drug Enforcement Administration Drugs and Chemicals of Concern. (2010). Spice – cannabinoids. Available at: http://www.deadiversion.usdoj.gov/drugs_concern/spice/index.html [accessed April 23, 2011].
Vardakou, I., Pistos, C., and Spiliopoulou, C. H. (2010). Spice drugs as a new trend: mode of action, identification and legislation. Toxicol. Lett. 197, 157–162.
Vardakou, I., Pistos, C., and Spiliopoulou, C. H. (2011). Drugs for youth via Internet and the example of mephedrone. Toxicol. Lett. 201, 191–195.
Vearrier, D., and Osterhoudt, K. C. (2010). A teenager with agitation: higher than she should have climbed. Pediatr. Emerg. Care 26, 462–465.
Weissman, A., Milne, M., and Melvin, M. S. Jr. (1982). Cannabimimetic activity from CP-47,497, a derivative of 3-phenylcyclohexanol. J. Pharmacol. Exp. Ther. 223, 516–523.
Westphal, F., Junge, T., Sonnichsen, F., Rosner, P., and Schaper, J. (2010). Ein neuer Wirkstoff in SPICE-artigen Kräutermischungen: Charakterisierung von JWH-250, seinen Methyl-und Trimethylsilylderivaten (A new compound in herbal mixtures: characterisation of JWH-250, its methyl- and trimethylsilyl-derivatives). Toxichem. Krimtech. 77, 46–58.
Westphal, F., Sönnichsen, F. D., and Thiemt, S. (2011). Identification of 1-butyl-3-(1-(4-methyl)naphthoyl)indole in a herbal mixture. Forensic Sci. Int. doi: 10.1016/j.forsciint.2011.03.031. [Epub ahead of print].
Wiley, J. L., Compton, D. R., Dai, D., Lainton, J. A., Phillips, M., Huffman, J. W., and Martin, B. R. (1998). Structure-activity relationships of indole- and pyrrole-derived cannabinoids. J. Pharmacol. Exp. Ther. 285, 995–1004.
Wintermeyer, A., Möller, I., Thevis, M., Jübner, M., Beike, J., Rothschild, M. A., and Bender, K. (2010). In vitro phase I metabolism of the synthetic cannabimimetic JWH-018. Anal. Bioanal. Chem. 398, 2141–2153.
World Anti-Doping Agency (WADA). (2011). Available at: http://www.wada-ama.org/rtecontent/document/2010_Prohibited_List_FINAL_EN_ Web.pdf [accessed April 5, 2011].
Zawilska, J. B. (2011). “Legal highs” – new players in the old drama. Curr. Drug Abuse Rev. 4, 122–130.
Zhang, Q., Ma, P., Cole, R. B., and Wang, G. (2006). Identification of in vitro metabolites of JWH-015, an aminoalkylindole agonist for the peripheral cannabinoid receptor (CB2) by HPLC-MS/MS. Anal. Bioanal. Chem. 386, 1345–1355.
Zimmermann, U. S., Winkelmann, P. R., Pilhatsch, M., Nees, J. A., Spanagel, R., and Schulz, K. (2009). Withdrawal phenomena and dependence syndrome after the consumption of “spice gold.” Dtsch. Artzebl. Int. 106, 464–467.
Zuardi, A. W., Crippa, J. A., Hallak, J. E., Moreira, F. A., and Guimarães, F. S. (2006). Cannabidiol, a cannabis sativa constituent, as an antipsychotic drug. Braz. J. Med. Biol. Res. 39, 421–429.
Zuardi, A. W., Shirakawa, I., Finkelfarb, E., and Karniol, I. G. (1982). Action of cannabidiol on the anxiety and other effects produced by delta 9-THC in normal subjects. Psychopharmacology (Berl.) 76, 245–250.
Keywords: spice, designer drugs, synthetic cannabinoids, addiction, Internet, herbal blends, natural highs, cannabimimetics
Citation: Fattore L and Fratta W (2011) Beyond THC: the new generation of cannabinoid designer drugs. Front. Behav. Neurosci. 5:60. doi: 10.3389/fnbeh.2011.00060
Received: 24 June 2011;
Paper pending published: 01 August 2011;
Accepted: 24 August 2011;
Published online: 21 September 2011.
Edited by:
Viviana Trezza, University “Roma Tre,” ItalyReviewed by:
Fabrício A. Pamplona, Universidade Federal de Santa Catarina, BrazilGernot Riedel, University of Aberdeen, UK
Marco Bortolato, University of Southern California, USA
Copyright: © 2011 Fattore and Fratta. This is an open-access article subject to a non-exclusive license between the authors and Frontiers Media SA, which permits use, distribution and reproduction in other forums, provided the original authors and source are credited and other Frontiers conditions are complied with.
*Correspondence: Liana Fattore, Institute of Neuroscience – Cagliari @ Department of Neuroscience, National Research Council of Italy, Cittadella Universitaria di Monserrato, University of Cagliari, 09042 Monserrato (Cagliari), Italy. e-mail: lfattore@in.cnr.it