Adverse Events of Intravesical OnabotulinumtoxinA Injection between Patients with Overactive Bladder and Interstitial Cystitis—Different Mechanisms of Action of Botox on Bladder Dysfunction?
Abstract
:1. Introduction
2. Results
2.1. Demographics
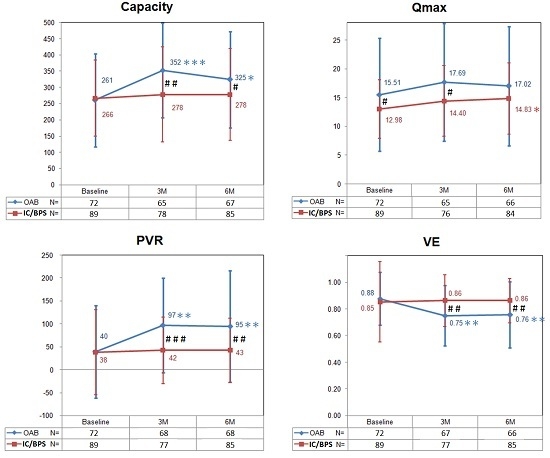
2.2. Effects of Botulinum Toxin A on Voiding Function
2.3. Adverse Events after Botulinum Toxin A Injections
3. Discussion
4. Materials and Methods
4.1. Patient Enrollment
4.2. Botulinum Toxin Injections
4.3. Patient Follow-Up
4.4. Statistical Analysis
5. Conclusions
Author Contributions
Conflicts of Interest
Abbreviations
| OAB | overactive bladder |
| UTI | urinary tract infection |
| BoNT-A | onabotulinumtoxinA |
| HR-QoL | health-related quality of life |
| AE | adverse event |
| AUR | acute urinary retention |
| PVR | postvoid residual |
| IC/BPS | interstitial cystitis/bladder pain syndrome |
| CISC | clean intermittent self-catheterization |
| AUA | American Urological Association |
| FBC | functional bladder capacity |
| Qmax | maximum flow rate |
| Pdet | detrusor pressure at Qmax |
| VE | voiding efficiency |
| DO | detrusor overactivity |
| HD | hydrodistention |
References
- Haylen, B.T.; Freeman, R.M.; Swift, S.E.; Cosson, M.; Davila, G.W.; Deprest, J.; Dwyer, P.L.; Fatton, B.; Kocjancic, E.; Lee, J.; et al. An international urogynecological association (iuga)/international continence society (ics) joint terminology and classification of the complications related directly to the insertion of prostheses (meshes, implants, tapes) and grafts in female pelvic floor surgery. Neurourol. Urodyn. 2011, 30, 2–12. [Google Scholar] [PubMed]
- Irwin, D.E.; Milsom, I.; Hunskaar, S.; Reilly, K.; Kopp, Z.; Herschorn, S.; Coyne, K.; Kelleher, C.; Hampel, C.; Artibani, W.; et al. Population-based survey of urinary incontinence, overactive bladder, and other lower urinary tract symptoms in five countries: Results of the epic study. Eur. Urol. 2006, 50, 1306–1314. [Google Scholar] [CrossRef] [PubMed]
- Malmsten, U.G.; Molander, U.; Peeker, R.; Irwin, D.E.; Milsom, I. Urinary incontinence, overactive bladder, and other lower urinary tract symptoms: A longitudinal population-based survey in men aged 45–103 years. Eur. Urol. 2010, 58, 149–156. [Google Scholar] [CrossRef] [PubMed]
- Wein, A.J.; Rackley, R.R. Overactive bladder: A better understanding of pathophysiology, diagnosis and management. J. Urol. 2006, 175, S5–S10. [Google Scholar] [CrossRef]
- Meng, E.; Lin, W.-Y.; Lee, W.-C.; Chuang, Y.-C. Pathophysiology of overactive bladder. LUTS Low. Urin. Tract Symptoms 2012, 4, 48–55. [Google Scholar] [CrossRef] [PubMed]
- Fowler, C.J.; Auerbach, S.; Ginsberg, D.; Hale, D.; Radziszewski, P.; Rechberger, T.; Patel, V.D.; Zhou, J.; Thompson, C.; Kowalski, J.W. Onabotulinumtoxina improves health-related quality of life in patients with urinary incontinence due to idiopathic overactive bladder: A 36-week, double-blind, placebo-controlled, randomized, dose-ranging trial. Eur. Urol. 2012, 62, 148–157. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Reduction of urgency severity is associated with long-term therapeutic effect after intravesical onabotulinumtoxin a injection for idiopathic detrusor overactivity. Neurourol. Urodyn. 2011, 30, 1497–1502. [Google Scholar] [CrossRef] [PubMed]
- Mohee, A.; Khan, A.; Harris, N.; Eardley, I. Long-term outcome of the use of intravesical botulinum toxin for the treatment of overactive bladder (oab). BJU Int. 2013, 111, 106–113. [Google Scholar] [CrossRef] [PubMed]
- Nitti, V.W.; Dmochowski, R.; Herschorn, S.; Sand, P.; Thompson, C.; Nardo, C.; Yan, X.; Haag-Molkenteller, C. Onabotulinumtoxina for the treatment of patients with overactive bladder and urinary incontinence: Results of a phase 3, randomized, placebo controlled trial. J. Urol. 2013, 189, 2186–2193. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C.; Liao, C.H.; Chung, S.D. Adverse events of intravesical botulinum toxin a injections for idiopathic detrusor overactivity: Risk factors and influence on treatment outcome. Eur. Urol. 2010, 58, 919–926. [Google Scholar] [CrossRef] [PubMed]
- Propert, K.J.; Schaeffer, A.J.; Brensinger, C.M.; Kusek, J.W.; Nyberg, L.M.; Landis, J.R. A prospective study of interstitial cystitis: Results of longitudinal followup of the interstitial cystitis data base cohort. The interstitial cystitis data base study group. J. Urol. 2000, 163, 1434–1439. [Google Scholar] [CrossRef]
- Butrick, C.W. Interstitial cystitis and chronic pelvic pain: New insights in neuropathology, diagnosis, and treatment. Clin. Obstet. Gynecol. 2003, 46, 811–823. [Google Scholar] [CrossRef] [PubMed]
- Hanno, P.M.; Erickson, D.; Moldwin, R.; Faraday, M.M. Diagnosis and treatment of interstitial cystitis/bladder pain syndrome: Aua guideline amendment. J. Urol. 2015, 193, 1545–1553. [Google Scholar] [CrossRef] [PubMed]
- Giannantoni, A.; Mearini, E.; Del Zingaro, M.; Proietti, S.; Porena, M. Two-year efficacy and safety of botulinum a toxin intravesical injections in patients affected by refractory painful bladder syndrome. Curr. Drug Deliv. 2010, 7, 1–4. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Repeated onabotulinumtoxin-a injections provide better results than single injection in treatment of painful bladder syndrome. Pain physician 2013, 16, E15–E23. [Google Scholar] [PubMed]
- Kuo, H.C.; Chancellor, M.B. Comparison of intravesical botulinum toxin type a injections plus hydrodistention with hydrodistention alone for the treatment of refractory interstitial cystitis/painful bladder syndrome. BJU Int. 2009, 104, 657–661. [Google Scholar] [PubMed]
- Pinto, R.; Lopes, T.; Silva, J.; Silva, C.; Dinis, P.; Cruz, F. Persistent therapeutic effect of repeated injections of onabotulinum toxin a in refractory bladder pain syndrome/interstitial cystitis. J. Urol. 2013, 189, 548–553. [Google Scholar] [CrossRef] [PubMed]
- Gormley, E.A.; Lightner, D.J.; Burgio, K.L.; Chai, T.C.; Clemens, J.Q.; Culkin, D.J.; Das, A.K.; Foster, H.E., Jr.; Scarpero, H.M.; Tessier, C.D.; et al. Diagnosis and treatment of overactive bladder (non-neurogenic) in adults: Aua/sufu guideline. J. Urol. 2012, 188, 2455–2463. [Google Scholar] [CrossRef] [PubMed]
- Coyne, K.S.; Matza, L.S.; Kopp, Z.; Abrams, P. The validation of the patient perception of bladder condition (ppbc): A single-item global measure for patients with overactive bladder. Eur. Urol. 2006, 49, 1079–1086. [Google Scholar] [CrossRef] [PubMed]
- Propert, K.J.; Mayer, R.D.; Wang, Y.; Sant, G.R.; Hanno, P.M.; Peters, K.M.; Kusek, J.W. Responsiveness of symptom scales for interstitial cystitis. Urology 2006, 67, 55–59. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.P.; Radziszewski, P.; Borkowski, A.; Somogyi, G.T.; Boone, T.B.; Chancellor, M.B. Botulinum toxin a has antinociceptive effects in treating interstitial cystitis. Urology 2004, 64, 871–875, discussion 875. [Google Scholar] [CrossRef] [PubMed]
- Meng, J.; Wang, J.; Lawrence, G.; Dolly, J.O. Synaptobrevin i mediates exocytosis of cgrp from sensory neurons and inhibition by botulinum toxins reflects their anti-nociceptive potential. J. Cell Sci. 2007, 120, 2864–2874. [Google Scholar] [CrossRef] [PubMed]
- Lucioni, A.; Bales, G.T.; Lotan, T.L.; McGehee, D.S.; Cook, S.P.; Rapp, D.E. Botulinum toxin type a inhibits sensory neuropeptide release in rat bladder models of acute injury and chronic inflammation. BJU Int. 2008, 101, 366–370. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Kuo, H.C. Intravesical botulinum toxin a injections plus hydrodistension can reduce nerve growth factor production and control bladder pain in interstitial cystitis. Urology 2007, 70, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Liu, H.T.; Tyagi, P.; Chancellor, M.B.; Kuo, H.C. Urinary nerve growth factor level is increased in patients with interstitial cystitis/bladder pain syndrome and decreased in responders to treatment. BJU Int. 2009, 104, 1476–1481. [Google Scholar] [CrossRef] [PubMed]
- Chapple, C.; Sievert, K.D.; MacDiarmid, S.; Khullar, V.; Radziszewski, P.; Nardo, C.; Thompson, C.; Zhou, J.; Haag-Molkenteller, C. Onabotulinumtoxina 100 u significantly improves all idiopathic overactive bladder symptoms and quality of life in patients with overactive bladder and urinary incontinence: A randomised, double-blind, placebo-controlled trial. Eur. Urol. 2013, 64, 249–256. [Google Scholar] [CrossRef] [PubMed]
- Mangera, A.; Andersson, K.E.; Apostolidis, A.; Chapple, C.; Dasgupta, P.; Giannantoni, A.; Gravas, S.; Madersbacher, S. Contemporary management of lower urinary tract disease with botulinum toxin a: A systematic review of botox (onabotulinumtoxina) and dysport (abobotulinumtoxina). Eur. Urol. 2011, 60, 784–795. [Google Scholar] [CrossRef] [PubMed]
- Yokoyama, T.; Chancellor, M.B.; Oguma, K.; Yamamoto, Y.; Suzuki, T.; Kumon, H.; Nagai, A. Botulinum toxin type a for the treatment of lower urinary tract disorders. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2012, 19, 202–215. [Google Scholar] [CrossRef] [PubMed]
- Yoshida, M.; Miyamae, K.; Iwashita, H.; Otani, M.; Inadome, A. Management of detrusor dysfunction in the elderly: Changes in acetylcholine and adenosine triphosphate release during aging. Urology 2004, 63, 17–23. [Google Scholar] [CrossRef] [PubMed]
- Mukerji, G.; Yiangou, Y.; Grogono, J.; Underwood, J.; Agarwal, S.K.; Khullar, V.; Anand, P. Localization of m2 and m3 muscarinic receptors in human bladder disorders and their clinical correlations. J. Urol. 2006, 176, 367–373. [Google Scholar] [CrossRef]
- Faubion, S.S.; Shuster, L.T.; Bharucha, A.E. Recognition and management of nonrelaxing pelvic floor dysfunction. Mayo Clin. Proc. 2012, 87, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Kuo, Y.-C.; Kuo, H.-C. O’leary-Sant symptom index predicts the treatment outcome for onabotulinumtoxin a injections for refractory interstitial cystitis/bladder pain syndrome. Toxins 2015, 7, 2860–2871. [Google Scholar] [CrossRef] [PubMed]
- Karsenty, G.; Denys, P.; Amarenco, G.; De Seze, M.; Game, X.; Haab, F.; Kerdraon, J.; Perrouin-Verbe, B.; Ruffion, A.; Saussine, C.; et al. Botulinum toxin a (botox) intradetrusor injections in adults with neurogenic detrusor overactivity/neurogenic overactive bladder: A systematic literature review. Eur. Urol. 2008, 53, 275–287. [Google Scholar] [CrossRef] [PubMed]
- Chung, S.D.; Kuo, Y.C.; Kuo, H.C. Intravesical onabotulinumtoxina injections for refractory painful bladder syndrome. Pain physician 2012, 15, 197–202. [Google Scholar] [PubMed]
- Pinto, R.; Lopes, T.; Frias, B.; Silva, A.; Silva, J.A.; Silva, C.M.; Cruz, C.; Cruz, F.; Dinis, P. Trigonal injection of botulinum toxin a in patients with refractory bladder pain syndrome/interstitial cystitis. Eur. Urol. 2010, 58, 360–365. [Google Scholar] [CrossRef] [PubMed]
- Homma, Y.; Ueda, T.; Tomoe, H.; Lin, A.T.; Kuo, H.C.; Lee, M.H.; Lee, J.G.; Kim, D.Y.; Lee, K.S. Clinical guidelines for interstitial cystitis and hypersensitive bladder syndrome. Int. J. Urol. Off. J. Jpn. Urol. Assoc. 2009, 16, 597–615. [Google Scholar] [CrossRef] [PubMed]
- Jiang, Y.H.; Ong, H.L.; Kuo, H.C. Predictive factors of adverse events after intravesical suburothelial onabotulinumtoxina injections for overactive bladder syndrome-a real-life practice of 290 cases in a single center. Neurourol. Urodyn. 2015. [Google Scholar] [CrossRef] [PubMed]
- Kuo, H.C. Comparison of effectiveness of detrusor, suburothelial and bladder base injections of botulinum toxin a for idiopathic detrusor overactivity. J. Urol. 2007, 178, 1359–1363. [Google Scholar] [CrossRef] [PubMed]


| Group | OAB (N = 72) | IC/BPS (N = 89) | p |
|---|---|---|---|
| Age (years) | 49.15 ± 10.85 | 48.81 ± 11.81 | 0.777 |
| Functional bladder capacity (mL) | 351.43 ± 135.21 | 124.72 ± 76.91 | 0.000 * |
| Daytime frequency (times/day) | 34.05 ± 14.56 | 15.64 ± 7.88 | 0.000 * |
| Nocturia (times/night) | 8.05 ± 2.99 | 4.90 ± 4.93 | 0.009 * |
| Urgency (times/24 h) | 33.00 ± 17.87 | - | - |
| Urge urinary incontinence (times/24 h) | 10.57 ± 12.98 | - | - |
| Visual analogue scale | - | 5.43 ± 2.24 | - |
| Maximum flow rate (Qmax) (mL/s) | 15.73 ± 9.69 | 12.62 ± 5.48 | 0.012 * |
| Voided volume (mL) | 224.86 ± 122.24 | 244.41 ± 112.12 | 0.364 |
| Postvoid residual (mL) | 39.19 ± 100.28 | 38.01 ± 93.26 | 0.916 |
| Total bladder capacity (mL) | 260.93 ± 143.25 | 266.19 ± 117.15 | 0.800 |
| Voiding efficiency | 0.88 ± 0.20 | 0.85 ± 0.30 | 0.577 |
| First sensation of filling (mL) | 112.14 ± 68.90 | 117.33 ± 53.28 | 0.744 |
| Strong desire to void (mL) | 210.86 ± 120.41 | 197.45 ± 87.74 | 0.597 |
| Cystometric bladder capacity (mL) | 264.61 ± 145.28 | 274.72 ± 109.92 | 0.714 |
| Detrusor pressure at Qmax (cm H2O) | 27.49 ± 13.60 | 19.19 ± 10.65 | 0.000 * |
| Adverse Events | OAB (%) | IC/BPS (%) | p |
|---|---|---|---|
| Hematuria | 7 (9.7) | 0 (0) | 0.003 a |
| UTI | 20 (27. 8) | 6 (6.7) | 0.000 a |
| Straining to void | 6 (8.3) | 27 (30.3) | 0.001 a |
| PVR > 200 mL | 23 (31.9) | 6 (6.7) | 0.000 a |
| AUR | 1 (1.4) | 0 (0) | 0.265 |
| Any | 42 (58.3) | 38 (42.7) | 0.048 b |
© 2016 by the authors; licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons by Attribution (CC-BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kuo, Y.-C.; Kuo, H.-C. Adverse Events of Intravesical OnabotulinumtoxinA Injection between Patients with Overactive Bladder and Interstitial Cystitis—Different Mechanisms of Action of Botox on Bladder Dysfunction? Toxins 2016, 8, 75. https://doi.org/10.3390/toxins8030075
Kuo Y-C, Kuo H-C. Adverse Events of Intravesical OnabotulinumtoxinA Injection between Patients with Overactive Bladder and Interstitial Cystitis—Different Mechanisms of Action of Botox on Bladder Dysfunction? Toxins. 2016; 8(3):75. https://doi.org/10.3390/toxins8030075
Chicago/Turabian StyleKuo, Yuh-Chen, and Hann-Chorng Kuo. 2016. "Adverse Events of Intravesical OnabotulinumtoxinA Injection between Patients with Overactive Bladder and Interstitial Cystitis—Different Mechanisms of Action of Botox on Bladder Dysfunction?" Toxins 8, no. 3: 75. https://doi.org/10.3390/toxins8030075