Abstract
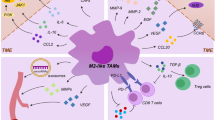
Tumor-associated macrophages (TAMs) derived from peripheral blood monocytes recruited into the renal cell carcinoma (RCC) microenvironment. In response to inflammatory stimuli, macrophages undergo M1 (classical) or M2 (alternative) activation. M1 cells produce high levels of inflammatory cytokines, such as tumor necrosis factor-α, interleukin (IL)-12, IL-23 and IL-6, while M2 cells produce anti-inflammatory cytokines, such as IL-10, thus contributing to RCC-related immune dysfunction. The presence of extensive TAM infiltration in RCC microenvironment contributes to cancer progression and metastasis by stimulating angiogenesis, tumor growth, and cellular migration and invasion. Moreover, TAMs are involved in epithelial–mesenchymal transition of RCC cancer cells and in the development of tumor resistance to targeted agents. Interestingly, macrophage autophagy seems to play an important role in RCC. Based on this scenario, TAMs represent a promising and effective target for cancer therapy in RCC. Several strategies have been proposed to suppress TAM recruitment, to deplete their number, to switch M2 TAMs into antitumor M1 phenotype and to inhibit TAM-associated molecules. In this review, we summarize current data on the essential role of TAMs in RCC angiogenesis, invasion, impaired anti-tumor immune response and development of drug resistance, thus describing the emerging TAM-centered therapies for RCC patients.


Similar content being viewed by others
References
Oliver RT, Nethersell AB, Bottomley JM (1989) Unexplained spontaneous regression and alpha-interferon as treatment for metastatic renal carcinoma. Br J Urol 63:128–131
Vogelzang NJ, Priest ER, Borden L (1992) Spontaneous regression of histologically proved pulmonary metastases from renal cell carcinoma: a case with 5-year follow-up. J Urol 148:1247–1248
Alexander RB, Fitzgerald EB, Mixon A, Carter CS, Jakobsen M, Cohen PA et al (1995) Helper T cells infiltrating human renal cell carcinomas have the phenotype of activated memory-like T lymphocytes. J Immunother Emphas Tumor Immunol 17:39–46
Komohara Y, Hasita H, Ohnishi K, Fujiwara Y, Suzu S, Eto M et al (2011) Macrophage infiltration and its prognostic relevance in clear cell renal cell carcinoma. Cancer Sci 102:1424–1431
Noessner E, Brech D, Mendler AN, Masouris I, Schlenker R, Prinz PU (2012) Intratumoral alterations of dendritic-cell differentiation and CD8+ T-cell anergy are immune escape mechanisms of clear cell renal cell carcinoma. Oncoimmunology 1:1451–1453
Figel AM, Brech D, Prinz PU, Lettenmeyer UK, Eckl J, Turqueti-Neves A et al (2011) Human renal cell carcinoma induces a dendritic cell subset that uses T-cell crosstalk for tumor-permissive milieu alterations. Am J Pathol 129:436–451
Zhang QW, Liu L, Gong CY, Shi HS, Zeng YH, Wang XZ et al (2012) Prognostic significance of tumor-associated macrophages in solid tumor: a meta-analysis of the literature. PLoS One 7:e50946
Hemmerlein B, Markus A, Wehner M, Kugler A, Zschunke F, Radzum HJ (2000) Expression of acute and late-stage inflammatory antigens, c-fms, CSF-1, and human monocytic serine esterase 1, in tumor-associated macrophages of renal cell carcinomas. Cancer Immunol Immunother 49:485–492
Mantovani A, Sozzani S, Locati M, Allavena P, Sica A (2002) Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol 23:549–555
Toge H, Inagaki T, Kojimoto Y, Shinka T, Hara I (2009) Angiogenesis in renal cell carcinoma: the role of tumor-associated macrophages. Int J Urol 16:801–807
Hemmerlein B, Scherbening J, Kugler A, Radzun HJ (2000) Expression of VCAM-1, ICAM-1, E- and P-selectin and tumour-associated macrophages in renal cell carcinoma. Histopathology 37:78–83
Ruffel B, Affara NI, Coussens LM (2012) Differential macrophage programming in the tumor microenvironment. Trends Immunol 33:119–126
Hao NB, Lu MH, Fan YH, Cao YL, Zhang ZR, Yang SM (2012) Macrophages in tumor microenvironments and the progression of tumors. Clin Dev Immunol 2012:948098. doi:10.1155/2012/948098
Li C, Liu B, Dai Z, Tao Y (2011) Knockdown of VEGF receptor-1 (VEGFR-1) impairs macrophage infiltration, angiogenesis and growth of clear cell renal cell carcinoma. Cancer Biol Ther 12:872–880
Rolny C, Mazzone M, Tugues S, Laoui D, Johansson I, Coulon C et al (2011) HRG inhibits tumor growth and metastasis by inducing macrophage polarization and vessel normalization through downregulation of PIGF. Cancer Cell 19:31–44
Fischer C, Jonckx B, Mazzone M, Zacchigna S, Loges S, Pattarini L et al (2007) Anti-PIGF inhibits growth of VEGF(R)-inhibitor-resistant tumors without affecting healthy vessels. Cell 131:463–475
Bedke J, Hemmerlein B, Perske C, Gross A, Heuser M (2010) Tumor-associated macrophages in clear cell renal cell carcinoma express both gastrin-releasing peptide and its receptor: a possible modulatory role of immune effectors cells. World J Urol 28:335–341
Rius J, Guma M, Schachtrup C, Akassoglou K, Zinkernagel AS, Nizet V et al (2008) NF-kappaB links innate immunity to the hypoxic response through transcriptional regulation of HIF-1alpha. Nature 453:807–811
Mosser DM, Edwards JP (2008) Exploring the full spectrum of macrophage activation. Nat Rev Immunol 8:958–969
Biswas SK, Allavena P, Mantovani A (2013) Tumor-associated macrophages: functional diversity, clinical significance, and open questions. Semin Immunopathol. doi:10.1007/s00281-013-0367-7
Allavena P, Sica A, Solinas G, Porta C, Mantovani A (2007) The inflammatory micro-environment in tumor progression: the role of tumor-associated macrophages. Crit Rev Oncol Hematol 66:1–9
Mantovani A, Sica A, Sozzani S, Allavena P, Vecchi A, Locati M (2004) The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol 25:677–686
Martinez FO, Gordon S, Locati M, Mantovani A (2006) Transcriptional profiling of the human monocyte-to-macrophage differentiation and polarization: new molecules and patterns of gene expression. J Immunol 177:7303–7311
Biswas SK, Mantovani A (2012) Orchestration of metabolism by macrophages. Cell Metab 15:432–437
Biswas SK, Mantovani A (2010) Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol 11:889–896
Yoshida N, Ikemoto S, Narita K, Sugimura K, Wada S, Yasumoto R et al (2002) Interleukin-6, tumour necrosis factor alpha and interleukin-1beta in patients with renal cell carcinoma. Br J Cancer 86:1396–1400
Yanase M, Tsukamoto T, Kumamoto Y (1995) Cytokines modulate in vitro invasiveness of renal cell carcinoma cells through action on the process of cell attachment to endothelial cells. J Urol 153:844–848
Dannenmann SR, Thielicke J, Stöckli M, Matter C, von Boehmer L, Cecconi V et al (2013) Tumor-associated macrophages subvert T-cell function and correlate with reduced survival in clear cell renal cell carcinoma. Oncoimmunology 2:e23562
Riches DW, Chan ED, Winston BW (1996) TNF-alpha-induced regulation and signalling in macrophages. Immunobiology 195:477–490
Balkwill F (2006) TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev 25:409–416
Balkwill F (2009) Tumour necrosis factor and cancer. Nat Rev Cancer 9:361–371
Falkensammer C, Jöhrer K, Gander H, Ramoner R, Putz T, Rahm A et al (2006) IL-4 inhibits the TNF-alpha induced proliferation of renal cell carcinoma (RCC) and cooperates with TNF-alpha to induce apoptotic and cytokine responses by RCC: implications for antitumor immune responses. Cancer Immunol Immunother 55:1228–1237
Ho MY, Tang SJ, Chuang MJ, Cha TL, Li JY, Sun GH et al (2012) TNF-α induces epithelial-mesenchymal transition of renal cell carcinoma cells via a GSK3β-dependent mechanism. Mol Cancer Res 10:1109–1119
Boström AK, Möller C, Nilsson E, Elfving P, Axelson H, Johansson ME (2012) Sarcomatoid conversion of clear cell renal cell carcinoma in relation to epithelial-to-mesenchymal transition. Hum Pathol 43:708–719
Kominsky SL, Doucet M, Brady K, Weber KL (2007) TGF-beta promotes the establishment of renal cell carcinoma bone metastasis. J Bone Miner Res 22:37–44
Sjölund J, Boström AK, Lindgren D, Manna S, Moustakas A, Ljungberg B et al (2011) The notch and TGF-β signaling pathways contribute to the aggressiveness of clear cell renal cell carcinoma. PLoS One 6:e23057. doi:10.1371/journal.pone.0023057
Paule B (2001) Interleukin-6 and bone metastasis of renal cancer: molecular bases and therapeutic implications. Prog Urol 11:368–375
Fitzgerald JP, Nayak B, Shanmugasundaram K, Friedrichs W, Sudarshan S, Eid AA et al (2012) Nox4 mediates renal cell carcinoma cell invasion through hypoxia-induced interleukin 6- and 8- production. PLoS One 7:e30712. doi:10.1371/journal.pone.0030712
Horiguchi A, Oya M, Marumo K, Murai M (2002) STAT3, but not ERKs, mediates the IL-6-induced proliferation of renal cancer cells, ACHN and 769P. Kidney Int 61:926–938
Porta C, Paglino C, Imarisio I, Ganini C, Sacchi L, Quaglini S et al (2013) Changes in circulating pro-angiogenic cytokines, other than VEGF, before progression to sunitinib therapy in advanced renal cell carcinoma patients. Oncology 84:115–122. doi:10.1159/000342099
Ikemoto S, Yoshida N, Narita K, Wada S, Kishimoto T, Sugimura K et al (2003) Role of tumor-associated macrophages in renal cell carcinoma. Oncol Rep 10:1843–1849
Daurkin I, Eruslanov E, Stoffs T, Perrin GQ, Algood C, Gilbert SM et al (2011) Tumor-associated macrophages mediate immunosuppression in the renal cancer microenvironment by activating the 15-lipoxygenase-2 pathway. Cancer Res 71:6400–6409
Kim IY, Lee DH, Lee DK, Kim BC, Kim HT, Leach FS et al (2003) Decreased expression of bone morphogenetic protein (BMP) receptor type II correlates with insensitivity to BMP-6 in human renal cell carcinoma cells. Clin Cancer Res 9:6046–6051
Kwak C, Park YH, Kim IY, Moon KC, Ku JH (2007) Expression of bone morphogenetic proteins, the subfamily of the transforming growth factor-beta superfamily, in renal cell carcinoma. J Urol 178:1062–1107
Blish KR, Wang W, Willingham MC, Du W, Birse CE, Krishnan SR et al (2008) A human bone morphogenetic protein antagonist is down-regulated in renal cancer. Mol Biol Cell 19:457–464
Sivertsen EA, Huse K, Hystad ME, Kersten C, Smeland EB, Myklebust JH (2007) Inhibitory effects and target genes of bone morphogenetic protein 6 in Jurkat TAg cells. Eur J Immunol 37:2937–2948
Kersten C, Sivertsen EA, Hystad ME, Forfang L, Smeland EB, Myklebust JH (2005) BMP-6 inhibits growth of mature human B cells; induction of Smad phosphorylation and upregulation of Id1. BMC Immunol 6:9
Hong JH, Lee GT, Lee JH, Kwon SJ, Park SH, Kim SJ et al (2009) Effect of bone morphogenetic protein-6 on macrophages. Immunology 128:e442–e450
Kwon SJ, Lee GT, Lee JH, Kim WJ, Kim IY (2009) Bone morphogenetic protein-6 induces the expression of inducible nitric oxide synthase in macrophages. Immunology 128:e758–e765
Lee JH, Lee GT, Woo SH, Ha YS, Kwon SJ, Kim WJ et al (2013) BMP-6 in Renal Cell Carcinoma Promotes Tumor Proliferation through IL-10-Dependent M2 Polarization of Tumor-Associated Macrophages. Cancer Res. doi:10.1158/0008-5472.CAN-12-4563
Yang Z, Klionsky DJ (2010) Mammalian autophagy: core molecular machinery and signalling regulation. Curr Opin Cell Biol 22:124–131
Chen Y, Klionsky DJ (2011) The regulation of autophagy—unanswered questions. J Cell Science 124:161–170
Mizushima N, Levine B, Cuervo AM, Klionsky DJ (2008) Autophagy fights disease through cellular self-digestion. Nature 451:1069–1075
Jacquel A, Obba S, Solary E, Auberger P (2012) Proper macrophagic differentiation requires both autophagy and caspase activation. Autophagy 8:1141–1143
Lin JC, Liu CL, Lee JJ, Liu TP, Ko WC, Huang YC et al (2013) Sorafenib induces autophagy and suppresses activation of human macrophage. Int Immunopharmacol 15:333–339
Donskov F, Hokland M, Marcussen N, Torp Madsen HH, von der Maase H (2006) Monocytes and neutrophils as ‘bad guys’ for the outcome of interleukin-2 with and without histamine in metastatic renal cell carcinoma–results from a randomised phase II trial. Br J Cancer 94:218–226
Perez-Gracia JL, Prior C, Guillén-Grima F, Segura V, Gonzalez A, Panizo A et al (2009) A. Identification of TNF-alpha and MMP-9 as potential baseline predictive serum markers of sunitinib activity in patients with renal cell carcinoma using a human cytokine array. Br J Cancer 101:1876–1883
Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE et al (2010) Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med 207:2455–2467
Zhang W, Zhu XD, Sun HC, Xiong YQ, Zhuang PY, Xu HX et al (2010) Depletion of tumor-associated macrophages enhances the effect of sorafenib in metastatic liver cancer models by antimetastatic and antiangiogenic effects. Clin Cancer Res 16:3420–3430
Edwards JP, Emens LA (2010) The multikinase inhibitor sorafenib reverses the suppression of IL-12 and enhancement of IL-10 by PGE2 in murine macrophages. Int Immunopharmacol 10:1220–1228
Tran HT, Liu Y, Zurita AJ, Lin Y, Baker-Neblett KL, Martin AM et al (2012) Prognostic or predictive plasma cytokines and angiogenic factors for patients treated with pazopanib for metastatic renal-cell cancer: a retrospective analysis of phase 2 and phase 3 trials. Lancet Oncol 13:827–837
Ko JS, Rayman P, Ireland J, Swaidani S, Li G, Bunting KD et al (2010) Direct and differential suppression of myeloid-derived suppressor cell subsets by sunitinib is compartmentally constrained. Cancer Res 70:3526–3536
Corzo CA, Condamine T, Lu L, Cotter MJ, Youn JI, Cheng P et al (2010) HIF-1α regulates function and differentiation of myeloid-derived suppressor cells in the tumor microenvironment. J Exp Med 207:2439–2453
Yang L, DeBusk LM, Fukuda K, Fingleton B, Green-Jarvis B, Shyr Y et al (2004) Expansion of myeloid immune suppressor Gr+ CD11b+ cells in tumor-bearing host directly promotes tumor angiogenesis. Cancer Cell 6:409–421
Sinha P, Clements VK, Bunt SK, Albelda SM, Ostrand-Rosenberg S (2007) Cross-talk between myeloid-derived suppressor cells and macrophages subverts tumor immunity toward a type 2 response. J Immunol 179:977–983
Sica A, Bronte V (2007) Altered macrophage differentiation and immune dysfunction in tumor development. J Clin Invest 117:1155–1166
Najjar YG, Finke JH (2013) Clinical perspectives on targeting of myeloid derived suppressor cells in the treatment of cancer. Front Oncol 3:49
Ko JS, Zea AH, Rini BI, Ireland JL, Elson P, Cohen P et al (2009) Sunitinib mediates reversal of myeloid-derived suppressor cell accumulation in renal cell carcinoma patients. Clin Cancer Res 15:2148–2157
Finke J, Ko J, Rini B, Rayman P, Ireland J, Cohen P (2011) MDSC as a mechanism of tumor escape from sunitinib mediated anti-angiogenic therapy. Int Immunopharmacol 11:856–861
Santoni M, Rizzo M, Burattini L, Farfariello V, Berardi R, Santoni G et al (2012) Recent Pat Antiinfect Drug Discov 7:104–110
Rogers TL, Holen I (2011) Tumor macrophages as potential target of bisphosphonates. J Transl Med 9:177
Vukanovic J, Isaacs JT (1995) Linomide inhibits angiogenesis, growth, metastasis, and macrophage infiltration within rat prostatic cancers. Cancer Res 55:1499–1504
Dineen SP, Lynn KD, Holloway SE, Miller AF, Sullivan JP, Shames DS et al (2008) Vascular endothelial growth factor receptor 2 mediates macrophage infiltration into orthotopic pancreatic tumors in mice. Cancer Res 68:4340–4346
Menke J, Kriegsmann J, Schimanski CC, Schwartz MM, Schwarting A, Kelley VR (2012) Autocrine CSF-1 and CSF-1 receptor coexpression promotes renal cell carcinoma growth. Cancer Res 72:187–200
Aharinejad S, Abraham D, Paulus P, Abri H, Hofmann M, Grossschmidt K et al (2002) Colony-stimulating factor-1 antisense treatment suppresses growth of human tumor xenografts in mice. Cancer Res 62:5317–5324
Ping YF, Yao XH, Jiang JY, Zhao LT, Yu SC, Jiang T et al (2011) The chemokine CXCL12 and its receptor CXCR4 promote glioma stem cell-mediated VEGF production and tumour angiogenesis via PI3K/AKT signalling. J Pathol 224:344–354
Bajetto A, Barbieri F, Dorcaratto A, Barbero S, Daga A, Porcile C et al (2006) Expression of CXC chemokine receptors 1-5 and their ligands in human glioma tissues: role of CXCR4 and SDF1 in glioma cell proliferation and migration. Neurochem Int 49:423–432
Cioli V, Ciarniello MG, Guglielmotti A, Luparini MR, Durando L, Martinelli B et al (1992) A new protein antidenaturant agent, bindarit, reduces secondary phase of adjuvant arthritis in rats. J Rheumatol 19:1735–1742
Zollo M, Di Dato V, Spano D, De Martino D, Liguori L, Marino N et al (2012) Targeting monocyte chemotactic protein-1 synthesis with bindarit induces tumor regression in prostate and breast cancer animal models. Clin Exp Metastasis 29:585–601
U’Ren L, Guth A, Kamstock D, Dow S (2010) Type I interferons inhibit the generation of tumor-associated macrophages. Cancer Immunol Immunother 59:587–598
Germano G, Frapolli R, Belgiovine C, Anselmo A, Pesce S, Liguori M et al (2013) Role of macrophage targeting in the antitumor activity of trabectedin. Cancer Cell 23:249–262
Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE (2009) Regulation of macrophage function in tumors: the multifaceted role of NF-κB. Blood 113:3139–3146
Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG et al (2008) “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med 205:1261–1268
Guiducci C, Vicari AP, Sangaletti S, Trinchieri G, Colombo MP (2005) Redirecting in vivo elicited tumor infiltrating macrophages and dendritic cells towards tumor rejection. Cancer Res 65:3437–3446
Nishihara K, Barth RF, Wilkie N, Lang JC, Oda Y, Kikuchi H et al (1995) Increased in vitro and in vivo tumoricidal activity of a macrophage cell line genetically engineered to express IFN-gamma, IL-4, IL-6, or TNF-alpha. Cancer Gene Ther 2:113–124
Horiguchi A, Asano T, Kuroda K, Sato A, Asakuma J, Ito K et al (2010) STAT3 inhibitor WP1066 as a novel therapeutic agent for renal cell carcinoma. Br J Cancer 102:1592–1599
Tyagi A, Singh RP, Ramasamy K, Raina K, Redente EF, Dwyer-Nield LD et al (2009) Growth inhibition and regression of lung tumors by silibinin: modulation of angiogenesis by macrophage-associated cytokines, and NF-κB and STAT3. Cancer Prev Res 2:74–83
Maisey N (2007) Antitumor necrosis factor (TNF-a) antibodies in the treatment of renal cell cancer. Cancer Invest 25:589–593
Larkin JM, Ferguson TR, Pickering LM, Edmonds K, James MG, Thomas K et al (2010) A phase I/II trial of sorafenib and infliximab in advanced renal cell carcinoma. Br J Cancer 103:1149–1153
Jin M, Xiao R, Wang J, Liu X, Liu Y, Xue Z et al (2013) Low concentrations of the recombinant toxin protein rLj-RGD3 suppress TNF-α-induced human renal carcinoma cell invasion. Acta Biochim Biophys Sin (Shanghai) 45:377–382
Lewēn S, Zhou H, Hu HD, Cheng T, Markowitz D, Reisfeld RA et al (2008) Legumain-based minigene vaccine targets the tumor stroma and suppresses breast cancer growth and angiogenesis. Cancer Immunol Immunother 57:507–515
Banciu M, Schiffelers RM, Fens MH, Metselaar JM, Storm G (2006) Anti-angiogenic effects of liposomal prednisolone phosphate on B16 melanoma in mice. J Control Release 113:1–8
Medical Research Council Renal Cancer Collaborators (1999) Interferon-alpha and survival in metastatic renal carcinoma: early results of randomised controlled trial. Lancet 353:14–17
Rosenberg SA, Lotze MT, Yang JC, Topalian SL, Chang AE, Schwartzentruber DJ et al (1993) Prospective randomized trial of high-dose interleukin 2 alone or in conjunction with lymphokine-activated killer cells for the treatment of patients with advanced cancer. J Natl Cancer Inst 85:622–632
Fyfe G, Fisher RI, Rosenberg SA, Sznol M, Parkinson DR, Louie AC (1995) Results of treatment of 255 patients with metastatic renal cell carcinoma who received high-dose recombinant interleukin-2 therapy. J Clin Oncol 13:688–696
McDermott DF, Regan MM, Clark JI, Flaherty LE, Weiss GR, Logan TF et al (2005) Randomized phase III trial of high-dose interleukin-2 versus subcutaneous interleukin-2 and interferon in patients with metastatic renal cell carcinoma. J Clin Oncol 23:133–141
Yang JC, Sherry RM, Steinberg SM, Topalian SL, Schwartzentruber DJ, Hwu P et al (2003) Randomized study of high-dose and low-dose interleukin-2 in patients with metastatic renal cancer. J Clin Oncol 21:3127–3132
Shablak A, Sikand K, Shanks JH, Thistlethwaite F, Spencer-Shaw A, Hawkins RE (2011) High-dose interleukin-2 can produce a high rate of response and durable remissions in appropriately selected patients with metastatic renal cancer. J Immunother 34:107–112
Escudier B, Eisen T, Stadler WM, Szczylik C, Oudard S, Siebels M et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Motzer RJ, Hutson TE, Tomczak P, Michaelson D, Bukowski RM, Rixe O et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Hudes G, Carducci M, Tomczak P, Dutcher J, Figlin R, Kapoor A et al (2007) Temsirolimus, interferon alfa, or both for advanced renal cell carcinoma. N Engl J Med 356:2271–2281
Escudier B, Pluzanska A, Koralewski P, Ravaud A, Bracarda S, Szczylik C et al (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomized, double-blind phase III trial. Lancet 370:2103–2111
Motzer RJ, Escudier B, Oudard S, Hutson TE, Porta C, Bracarda S et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind randomised placebo-controlled phase III trial. Lancet 372:449–456
Rini BI, Halabi S, Rosenberg JE, Stadler WM, Vaena DA, Ou SS et al (2008) Bevacizumab plus interferon alfa compared with interferon alfa monotherapy in patients with metastatic renal cell carcinoma: CALGB 90206. J Clin Oncol 26:5422–5428
Sternberg CN, Davis ID, Mardiak J, Szczylik C, Lee E, Wagstaff J et al (2010) Pazopanib in locally advanced or metastatic renal cell carcinoma: results of a randomized phase III trial. J Clin Oncol 28:1061–1068
Rini BI, Escudier B, Tomczack P, Kaprin A, Szczylik C, Hutson TE et al (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378:1931–1939
Motzer RJ, Nosov D, Eisen T, Bondarenko I, Lesovoy V, Lipatov O, et al (2012) Tivozanib versus sorafenib as initial targeted therapy for patients with advanced renal cell carcinoma: results from a phase III randomized, open-label, multicenter trial. J Clin Oncol 30: abstr 4501
Martinez FO, Sica A, Mantovani A, Locati M (2008) Macrophage activation and polarization. Front Biosci 13:453–461
Bingle L, Brown NJ, Lewis CE (2002) The role of tumourassociated macrophages in tumour progression: implications for new anticancer therapies. J Pathol 196:254–265
Edin S, Wikberg ML, Dahlin AM, Rutegård J, Öberg Å, Oldenborg PA, Palmqvist R (2012) The distribution of macrophages with a M1 or M2 phenotype in relation to prognosis and the molecular characteristics of colorectal cancer. PLoS One 7:e47045. doi:10.1371/journal.pone.0047045
Acknowledgments
Many thanks to Professor Alberto Mantovani, Scientific Director of Istituto Clinico Humanitas, Milan, Italy, for his collaboration during the preparation of this manuscript. Particular thanks to Francesca Tartari for her precious help in drafting the manuscript.
Conflict of interest
We declare to have no conflict of interest.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Santoni, M., Massari, F., Amantini, C. et al. Emerging role of tumor-associated macrophages as therapeutic targets in patients with metastatic renal cell carcinoma. Cancer Immunol Immunother 62, 1757–1768 (2013). https://doi.org/10.1007/s00262-013-1487-6
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s00262-013-1487-6