Abstract
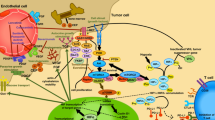
Antiangiogenic therapy with vascular endothelial growth factor (VEGF) inhibitors is the current first-line treatment in metastatic renal cell carcinoma (mRCC). Immunotherapy with checkpoint inhibitor has been recently added to the armamentarium of mRCC treatment. These therapies are based on treatment with antibodies that block programmed cell death-1 (PD-1), programmed cell death ligand 1 (PD-L1) pathways, demonstrating impressive response rates and improved survival in several tumour types. So far, nivolumab is the only approved anti-PD-1 monoclonal antibody after VEGF therapy in mRCC. According to preclinical and clinical studies, combination therapies with VEGF- and checkpoint inhibitors have synergistic effect achieving improved response rates. However, toxicity in some combinations is high. In this article, we present a review of the ongoing trials with these drug combinations for RCC.

Similar content being viewed by others
References
Znaor A, Lortet-Tieulent J, Laversanne M, Jemal A, Bray F (2015) International variations and trends in renal cell carcinoma incidence and mortality. Eur Urol 67(3):519–530
Siegel RL, Miller KD, Jemal A (2016) Cancer statistics, 2016. CA Cancer J Clin 66:7–30
Ferlay J, Steliarova-Foucher E, Lortet-Tieulent J et al (2013) Cancer incidence and mortality patterns in Europe: estimates for 40 countries in 2012. Eur J Cancer 49:1374–1403
Ljungberg B, Bensalah K, Canfield S et al (2015) EAU guidelines on RCC-an update 2014. Eur Urol 67(5):913–924
Escudier B, Porta C, Schmidinger M et al (2016) Renal cell carcinoma: ESMO Clinical Practice Guidelines for diagnosis, treatment and follow-up. Ann Oncol 27(5):58–68
Latif F, Tory K, Gnarra J et al (1993) Identification of the von Hippel-Lindau disease tumor suppressor gene. Science 260:1317–1320
Linehan WM, Pinto PA, Srinivasan R et al (2007) Identification of the genes for kidney cancer: opportunity for disease-specific targeted therapeutics. Clin Cancer Res 15(13):671–679
Braren V, Taylor JN, Pace W et al (1974) Regression of metastatic renal carcinoma following nephrectomy. Urology 3:777–778
Fisher RI, Rosenberg SA, Fyfe G et al (2000) Long-term survival update for high-dose recombinant interleukin-2 in patients with renal cell carcinoma. Cancer J Sci Am 6(1):55–57
Escudier B, Pluzanska A, Koralewski P et al (2007) Bevacizumab plus interferon alfa-2a for treatment of metastatic renal cell carcinoma: a randomised, double-blind phase III trial. Lancet 22(370):2103–2111
Kandalaft LE, Motz GT, Busch J, Coukos G (2011) Angiogenesis and the tumor vasculature as antitumor immune modulators: the role of vascular endothelial growth factor and endothelin. Curr Top Microbiol Immunol 344:129–148
Mikucki ME, Fisher DT, Matsuzaki J et al (2015) Non-redundant requirement for CXCR3 signaling during tumoricidal T cell trafficking across tumor vascular checkpoints. Nat Commun 6:7458
Hodi FS, Mihm MC, Soiffer RJ et al (2003) Biologic activity of cytotoxic T lymphocyte-associated antigen 4 antibody blockade in previously vaccinated metastatic melanoma and ovarian carcinoma patients. Proc Natl Acad Sci USA 100(8):4712–4717
Jayson GC, Kerbel R, Ellis LM, Harris AL (2016) Antiangiogenic therapy in oncology: current status and future directions. Lancet 388(10043):518–529
Griffioen AW, Mans LA, de Graaf AM et al (2012) Rapid angiogenesis onset after discontinuation of sunitinib treatment of renal cell carcinoma patients. Clin Cancer Res 18(14):3961–3971
Yang JC, Haworth L, Sherry RM et al (2003) A randomized trial of bevacizumab, an anti-vascular endothelial growth factor antibody, for metastatic renal cancer. N Engl J Med 349:427–434
Motzer RJ, Hutson TE, Tomczak P et al (2007) Sunitinib versus interferon alfa in metastatic renal-cell carcinoma. N Engl J Med 356:115–124
Motzer RJ, Escudier B, Oudard S et al (2008) Efficacy of everolimus in advanced renal cell carcinoma: a double-blind, randomised, placebo-controlled phase III trial. Lancet 372:449–456
Escudier B, Eisen T, Stadler WM et al (2007) Sorafenib in advanced clear-cell renal-cell carcinoma. N Engl J Med 356:125–134
Motzer RJ, Hutson TE, Cella D et al (2013) Pazopanib versus sunitinib in metastatic renal-cell carcinoma. N Engl J Med 369:722–731
Rini BI, Escudier B, Tomczak P et al (2011) Comparative effectiveness of axitinib versus sorafenib in advanced renal cell carcinoma (AXIS): a randomised phase 3 trial. Lancet 378:1931–1939
Schreiber RD, Old LJ, Smyth MJ (2011) Cancer immunoediting: integrating immunity’s roles in cancer suppression and promotion. Science 331(6024):1565–1570
Topalian SL, Drake CG, Pardoll DM (2015) Immune checkpoint blockade: a common denominator approach to cancer therapy. Cancer Cell 27(4):450–461
Kirkwood JM, Butterfield LH, Tarhini AA et al (2012) Immunotherapy of cancer in 2012. CA Cancer J Clin 62(5):309–335
Pardoll DM (2012) The blockade of immune checkpoints in cancer immunotherapy. Nat Rev Cancer 12(4):252–264
Brahmer JR, Tykodi SS, Chow LQ et al (2012) Safety and activity of anti-PD-L1 antibody in patients with advanced cancer. N Engl J Med 366:2455–2465
Motzer RJ, Escudier B, McDermott DF et al (2015) Nivolumab versus Everolimus in Advanced Renal-Cell Carcinoma. N Engl J Med 373:1803–1813
Tartour E, Pere H, Maillere B et al (2011) Angiogenesis and immunity: a bidirectional link potentially relevant for the monitoring of antiangiogenic therapy and the development of novel therapeutic combination with immunotherapy. Cancer Metastasis Rev 30(1):83–95
Vanneman M, Dranoff G (2012) Combining immunotherapy and targeted therapies in cancer treatment. Nat Rev Cancer 12(4):237–251
Guislain A, Gadiot J, Kaiser A et al (2015) Sunitinib pretreatment improves tumor-infiltrating lymphocyte expansion by reduction in intratumoral content of myeloid-derived suppressor cells in human renal cell carcinoma. Cancer Immunol Immunother 2015. Cancer Immunol Immunother 64(10):1241–1250
Liu XD, Hoang A, Zhou L et al (2015) Resistance to antiangiogenic therapy is associated with an immunosuppressive tumor microenvironment in metastatic renal cell carcinoma. Cancer Immunol Res 3(9):1017–1029
Wallin JJ, Bendell JC, Funke R et al (2016) Atezolizumab in combination with bevacizumab enhances antigen-specific T-cell migration in metastatic renal cell carcinoma. Nat Commun 30(7):12624. doi:10.1038/ncomms12624
Amin A, Plimack ER, Infante JR et al (2014) Nivolumab (anti-PD-1; BMS-936558, ONO-4538) in combination with sunitinib or pazopanib in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 32(5):5010. doi:10.1200/JCO.2013.54.6911
Dudek AZ, Sica RA, Sidani A et al (2016) Phase Ib study of pembrolizumab in combination with bevacizumab for the treatment of metastatic renal cell carcinoma: Big Ten Cancer Research Consortium BTCRC-GU14-003. J Clin Oncol 34(2S):559
ClinicalTrials.gov (NCT02298959) Pembrolizumab and Ziv-aflibercept in Treating Patients With Advanced Solid Tumors
Taylor M, Dutcus CE, Schmidt E et al (2016) A phase 1b trial of lenvatinib (LEN) plus pembrolizumab (PEM) in patients with selected solid tumors. Ann Oncol 27(6):266–295
Clinicaltrials.gov (NCT02811861) Lenvatinib/Everolimus or Lenvatinib/Pembrolizumab Versus Sunitinib Alone as Treatment of Advanced Renal Cell Carcinoma
McDermott DF, Infante JR, Chowdhury S et al (2015) A phase I/II study to assess the safety and efficacy of pazopanib (paz) and pembrolizumab (pembro) in patients (pts) with untreated advanced renal cell carcinoma (aRCC). Eur J Cancer 51:519–520
Atkins MB, Plimack ER, Puzanov I et al (2016) Axitinib in combination with pembrolizumab in patients (pts) with advanced renal cell carcinoma (aRCC): preliminary safety and efficacy results. Ann Oncol 27(6):266–295
Larkin JMG, Gordon MS, Thistlethwaite F, et al (2016) Avelumab (MSB0010718C; anti-PD-L1) in combination with axitinib as first-line treatment for patients with advanced renal cell carcinoma. J Clin Oncol 34(Abstract TPS4580)
Clinicaltrials.gov (KEYNOTE-426 NCT02853331) Study to Evaluate the Efficacy and Safety of Pembrolizumab (MK-3475) in Combination With Axitinib Versus Sunitinib Monotherapy in Participants With Renal Cell Carcinoma (MK-3475-426/KEYNOTE-426)
Clinicaltrials.gov (JAVELIN Renal 101 NCT02684006) A Study of Avelumab With Axitinib Versus Sunitinib In Advanced Renal Cell Cancer (JAVELIN Renal 101)
Sznol M, McDermott DF, Fields JS et al (2015) Phase Ib evaluation of MPDL3280A (anti-PDL1) in combination with bevacizumab (bev) in patients (pts) with metastatic renal cell carcinoma (mRCC). J Clin Oncol 33(7):410
McDermott DF, Atkins MB, Motzer RJ, et al (2017) A phase II study of atezolizumab (atezo) with or without bevacizumab (bev) versus sunitinib (sun) in untreated metastatic renal cell carcinoma (mRCC) patients (pts). Paper presented at: 2017 Genitourinary Cancers Symposium; February 16–18, 2017; Orlando, FL
Clinicaltrials.gov (IMmotion 151 NCT02420821) A Study of Atezolizumab in Combination With Bevacizumab Versus Sunitinib in Participants With Untreated Advanced Renal Cell Carcinoma
Rini BI, Stein M, Shannon P et al (2011) Phase 1 dose-escalation trial of tremelimumab plus sunitinib in patients with metastatic renal cell carcinoma. Cancer 117:758–767
ClinicalTrials.gov (NCT02724878) Study of Atezolizumab + Bevacizumab in Patients With Advanced Non-Clear Cell Renal Cell Carcinoma
ClinicalTrials.gov (NCT02819596) MEDI4736 Combinations in Metastatic Renal Cell Carcinoma (CALYPSO)
Apolo A, Mortazavi A, Stein M, et al (2017) A phase I study of cabozantinib plus nivolumab (CaboNivo) and ipilimumab (CaboNivoIpi) in patients (pts) with refractory metastatic urothelial carcinoma (mUC) and other genitourinary (GU) tumors. J Clin Oncol 35, 2017 (suppl 6S; abstract 293)
Greef B, Eisen T (2016) Medical treatment of renal cancer: new horizons. Br J Cancer 115:505–516
Liu J, Blake SJ, Yong MC et al (2016) Improved efficacy of neoadjuvant compared to adjuvant immunotherapy to eradicate metastatic disease. Cancer Discov 6:1382–1399
ClinicalTrials.gov (NCT02212730) A Study Evaluating the Effect of Pembrolizumab (MK-3475) in Participants With Renal Cell Cancer (MK-3475-031)
ClinicalTrials.gov (NCT02575222) Study of Neoadjuvant Nivolumab in Patients With Non-metastatic Stage II-IV Clear Cell Renal Cell Carcinoma
ClinicalTrials.gov (NCT02446860) A Study of Anti-PD1 (Nivolumab) Therapy as Pre- and Post-operative Therapy in Metastatic Renal Cell Cancer (ADAPTeR) (ADAPTeR)
ClinicalTrials.gov (NCT02210117) Nivolumab vs Nivolumab + Bevacizumab vs Nivolumab + Ipilimumab in Metastatic Renal Cell Carcinoma (mRCC)
Massari F, Santoni M, Ciccarese C et al (2015) PD-1 blockade therapy in renal cell carcinoma: current studies and future promises. Cancer Treat Rev 41:114–121
Kasenda B, Larkin J, Gore M (2015) Immunotherapies in early and advanced renal cell cancer. Prog Tumor Res 42:1–10
Patel DN, Figlin RA, Kim HL (2016) Adjuvant treatment for renal cell carcinoma: Do we finally have a major breakthrough? Clin Adv Hematol Oncol 14(11):907–914
ClinicalTrials.gov (NCT03024996) A Study of Atezolizumab as Adjuvant Therapy in Participants With Renal Cell Carcinoma (RCC) at High Risk of Developing Metastasis Following Nephrectomy (IMmotion010)
Bex A, Albiges L, Ljungberg B et al (2016) Updated European Association of Urology Guidelines Regarding Adjuvant Therapy for Renal Cell Carcinoma. Eur Urol. doi:10.1016/j.eururo.2016.11.034
Acknowledgements
We would like to thank the European Urological Scholarship Programme for financially supporting the research fellowship of TK and NG at Antoni van Leeuwenhoek Hospital/the Netherlands Cancer Institute in Amsterdam, the Netherlands.
Financial disclosure
The authors declare that they have no relevant financial interests.
Conflict of interest
AB took part in advisory boards of Pfizer, Novartis, Ipsen, Eisai and Roche. He is the PI of the EORTC SURTIME trial sponsored in part by a Grant from Pfizer to the EORTC. LA has received consulting and advisory fees from BMS, Pfizer, Novartis, Sanofi, Amgen, Bristol-Myers Squibb, Bayer, and Cerulean; and research funding from Pfizer and Novartis. BE has received fees for serving on advisory boards from Pfizer, Novartis, Bristol-Myers Squibb, Exelixis, and Roche and lecture fees from Pfizer and Novartis. JH has consulted or has an advisory role for MSD Oncology, Pfizer, and Bristol-Myers Squibb, as well as gained research funding by MSD and Bristol-Myers Squibb. TP is a company consultant for Novartis, Pfizer, GSK, has received company speaker honoraria from Novartis, Pfizer, GSK, Genentech, performed trial participation for GSK, Pfizer, BMS, Genentech, Genetech, and received grants/research support from GSK, Pfizer, and Novartis. The other authors have no conflict of interest to declare.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Kuusk, T., Albiges, L., Escudier, B. et al. Antiangiogenic therapy combined with immune checkpoint blockade in renal cancer. Angiogenesis 20, 205–215 (2017). https://doi.org/10.1007/s10456-017-9550-0
Received:
Accepted:
Published:
Issue Date:
DOI: https://doi.org/10.1007/s10456-017-9550-0