Abstract
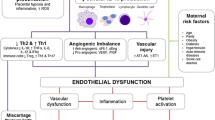
Preeclampsia (PE) is one of the leading causes of maternal morbidity and mortality worldwide. This disease is believed to occur in two stages with placental dysfunction in early pregnancy leading to maternal clinical findings after 20 weeks of gestation, as consequence of systemic inflammation, oxidative stress, and endothelial dysfunction. Much evidence suggests that PE women display an overshooting inflammatory response throughout pregnancy due to an unbalanced regulation of innate and adaptive immune responses. Recently, it has been suggested that dysregulation of endogenous protective pathways might be associated with PE etiopathogenesis. Resolution of inflammation is an active process coordinated by mediators from diverse nature that regulate key cellular events to restore tissue homeostasis. Inadequate or insufficient resolution of inflammation is believed to play an important role in the development of chronic inflammatory diseases, like PE. In this narrative review, we discuss possible pro-resolution pathways that might be compromised in PE women, which could be targets to novel therapeutic strategies in this disease.



Similar content being viewed by others
References
American College of Obstetricians and Gynecologists. Hypertension in pregnancy. Report of the American College of Obstetricians and Gynecologists’ Task Force on Hypertension in Pregnancy. Obstet Gynecol. 2013;122(5):1122–31.
Chaiworapongsa T, Chaemsaithong P, Yeo L, Romero R. Pre-eclampsia part 1: current understanding of its pathophysiology. Nat Rev Nephrol. 2014;10(8):466–80.
American College of Obstetricians and Gynecologists. ACOG practice bulletin. Diagnosis and management of preeclampsia and eclampsia. Number 33, January 2002. Obstet Gynecol. 2002;99(1):159–67.
von Dadelszen P, Magee LA, Roberts JM. Subclassification of preeclampsia. Hypertens Pregnancy. 2003;22(2):143–8.
Steegers EA, von Dadelszen P, Duvekot JJ, Pijnenborg R. Pre-eclampsia. Lancet. 2010;376(9741):631–44.
Raymond D, Peterson E. A critical review of early-onset and late-onset preeclampsia. Obstet Gynecol Surv. 2011;66(8):497–506.
Roberts JM, Hubel CA. The two stage model of preeclampsia: variations on the theme. Placenta. 2009;30(Suppl A):S32–7.
Lam C, Lim KH, Karumanchi SA. Circulating angiogenic factors in the pathogenesis and prediction of preeclampsia. Hypertension. 2005;46(5):1077–85.
Ahmed A, Ramma W. Unravelling the theories of pre-eclampsia: are the protective pathways the new paradigm? Br J Pharmacol. 2015;172(6):1574–86.
Cotechini T, Komisarenko M, Sperou A, Macdonald-Goodfellow S, Adams MA, Graham CH. Inflammation in rat pregnancy inhibits spiral artery remodeling leading to fetal growth restriction and features of preeclampsia. J Exp Med. 2014;211(1):165–79.
Matsubara K, Higaki T, Matsubara Y, Nawa A. Nitric oxide and reactive oxygen species in the pathogenesis of preeclampsia. Int J Mol Sci. 2015;16(3):4600–14.
Wallace JL, Ianaro A, Flannigan KL, Cirino G. Gaseous mediators in resolution of inflammation. Semin Immunol. 2015;27(3):227–33.
Chovatiya R, Medzhitov R. Stress, inflammation, and defense of homeostasis. Mol Cell. 2014;54(2):281–8.
Serhan CN, Savill J. Resolution of inflammation: the beginning programs the end. Nat Immunol. 2005;6(12):1191–7.
Serhan CN, Brain SD, Buckley CD, Gilroy DW, Haslett C, O’Neill LA, et al. Resolution of inflammation: state of the art, definitions and terms. FASEB J. 2007;21(2):325–32.
Headland SE, Norling LV. The resolution of inflammation: principles and challenges. Semin Immunol. 2015;27(3):149–60.
Sugimoto M, Sousa L, Pinho V, Perretti M, Teixeira M. Resolution of inflammation: what controls its onset? Front Immunol. 2016;160(7):1–18.
Vago JP, Tavares LP, Sugimoto MA, Lima GL, Galvão I, de Caux TR, et al. Proresolving actions of synthetic and natural protease inhibitors are mediated by Annexin A1. J Immunol. 2016;196(4):1922–32.
Odaka C, Mizuochi T, Yang J, Ding A. Murine macrophages produce secretory leukocyte protease inhibitor during clearance of apoptotic cells: implications for resolution of the inflammatory response. J Immunol. 2003;171(3):1507–14.
Titos E, Rius B, González-Périz A, López-Vicario C, Morán-Salvador E, Martínez-Clemente M, et al. Resolvin D1 and its precursor docosahexaenoic acid promote resolution of adipose tissue inflammation by eliciting macrophage polarization toward an M2-like phenotype. J Immunol. 2011;187(10):5408–18.
Fullerton JN, Gilroy DW. Resolution of inflammation: a new therapeutic frontier. Nat Rev Drug Discov. 2016;15(8):551–67.
Orsi NM. Cytokine networks in the establishment and maintenance of pregnancy. Hum Fertil (Camb). 2008;11(4):222–30.
Redman CW, Sacks GP, Sargent IL. Preeclampsia: an excessive maternal inflammatory response to pregnancy. Am J Obstet Gynecol. 1999;180(2 Pt 1):499–506.
Schminkey DL, Groer M. Imitating a stress response: a new hypothesis about the innate immune system’s role in pregnancy. Med Hypotheses. 2014;82(6):721–9.
Saito S, Nakashima A, Shima T, Ito M. Th1/Th2/Th17 and regulatory T-cell paradigm in pregnancy. Am J Reprod Immunol. 2010;63(6):601–10.
Li M, Piao L, Chen CP, Wu X, Yeh CC, Masch R, et al. Modulation of decidual macrophage polarization by macrophage colony-stimulating factor derived from first-trimester decidual cells: implication in preeclampsia. Am J Pathol. 2016;186(5):1258–66.
Lee CL, Guo Y, So KH, Vijayan M, Wong VH, Yao Y, et al. Soluble human leukocyte antigen G5 polarizes differentiation of macrophages toward a decidual macrophage-like phenotype. Hum Reprod. 2015;30(10):2263–74.
Perretti M, D’Acquisto F. Annexin A1 and glucocorticoids as effectors of the resolution of inflammation. Nat Rev Immunol. 2009;9(1):62–70.
Sugimoto MA, Vago JP, Teixeira MM, Sousa LP. Annexin A1 and the resolution of inflammation: modulation of neutrophil recruitment, apoptosis, and clearance. J Immunol Res. 2016;2016:8239258.
John CD, Gavins FN, Buss NA, Cover PO, Buckingham JC. Annexin A1 and the formyl peptide receptor family: neuroendocrine and metabolic aspects. Curr Opin Pharmacol. 2008;8(6):765–76.
John CD, Christian HC, Morris JF, Flower RJ, Solito E, Buckingham JC. Annexin 1 and the regulation of endocrine function. Trends Endocrinol Metab. 2004;15(3):103–9.
Eke Gungor H, Tahan F, Gokahmetoglu S, Saraymen B. Decreased levels of lipoxin A4 and annexin A1 in wheezy infants. Int Arch Allergy Immunol. 2014;163(3):193–7.
Williams SL, Milne IR, Bagley CJ, Gamble JR, Vadas MA, Pitson SM, et al. A proinflammatory role for proteolytically cleaved annexin A1 in neutrophil transendothelial migration. J Immunol. 2010;185(5):3057–63.
Tsao FH, Meyer KC, Chen X, Rosenthal NS, Hu J. Degradation of annexin I in bronchoalveolar lavage fluid from patients with cystic fibrosis. Am J Respir Cell Mol Biol. 1998;18(1):120–8.
Cooray SN, Gobbetti T, Montero-Melendez T, McArthur S, Thompson D, Clark AJ, et al. Ligand-specific conformational change of the G-protein-coupled receptor ALX/FPR2 determines proresolving functional responses. Proc Natl Acad Sci U S A. 2013;110(45):18232–7.
Planagumà A, Kazani S, Marigowda G, Haworth O, Mariani TJ, Israel E, et al. Airway lipoxin A4 generation and lipoxin A4 receptor expression are decreased in severe asthma. Am J Respir Crit Care Med. 2008;178(6):574–82.
Perucci LO, Carneiro FS, Ferreira CN, Sugimoto MA, Soriani FM, Martins GG, et al. Annexin A1 is increased in the plasma of preeclamptic women. PLoS One. 2015;10(9):e0138475.
Xu Z, Zhao F, Lin F, Xiang H, Wang N, Ye D, et al. Preeclampsia is associated with a deficiency of lipoxin A4, an endogenous anti-inflammatory mediator. Fertil Steril. 2014;102(1):282–90.e4.
Dong W, Yin L. Expression of lipoxin A4, TNFα and IL-1β in maternal peripheral blood, umbilical cord blood and placenta, and their significance in pre-eclampsia. Hypertens Pregnancy. 2014;33(4):449–56.
Behrouz GF, Farzaneh GS, Leila J, Jaleh Z, Eskandar KS. Presence of auto-antibody against two placental proteins, annexin A1 and vitamin D binding protein, in sera of women with pre-eclampsia. J Reprod Immunol. 2013;99(1–2):10–6.
Canzoneri BJ, Lewis DF, Groome L, Wang Y. Increased neutrophil numbers account for leukocytosis in women with preeclampsia. Am J Perinatol. 2009;26(10):729–32.
Gupta AK, Gebhardt S, Hillermann R, Holzgreve W, Hahn S. Analysis of plasma elastase levels in early and late onset preeclampsia. Arch Gynecol Obstet. 2006;273(4):239–42.
Salama RH, Fathalla MM, Mekki AR, Elsadek B-K. Implication of umbilical cord in preeclampsia. Med Princ Pract. 2011;20(2):124–8.
Sun M, Liu Y, Gibb W. Distribution of annexin I and II in term human fetal membranes, decidua and placenta. Placenta. 1996;17(2–3):181–4.
Leffler H, Carlsson S, Hedlund M, Qian Y, Poirier F. Introduction to galectins. Glycoconj J. 2004;19(7–9):433–40.
Sato S, St-Pierre C, Bhaumik P, Nieminen J. Galectins in innate immunity: dual functions of host soluble beta-galactoside-binding lectins as damage-associated molecular patterns (DAMPs) and as receptors for pathogen-associated molecular patterns (PAMPs). Immunol Rev. 2009;230(1):172–87.
Rubinstein N, Ilarregui JM, Toscano MA, Rabinovich GA. The role of galectins in the initiation, amplification and resolution of the inflammatory response. Tissue Antigens. 2004;64(1):1–12.
Arikawa T, Simamura E, Shimada H, Nakamura T, Hatta T, Shoji H. Significance of sugar chain recognition by galectins and its involvement in disease-associated glycosylation. Congenit Anom (Kyoto). 2014;54(2):77–81.
Vasta GR. Galectins as pattern recognition receptors: structure, function, and evolution. Adv Exp Med Biol. 2012;946:21–36.
Romaniuk MA, Negrotto S, Campetella O, Rabinovich GA, Schattner M. Identification of galectins as novel regulators of platelet signaling and function. IUBMB Life. 2011;63(7):521–7.
Blois SM, Conrad ML, Freitag N, Barrientos G. Galectins in angiogenesis: consequences for gestation. J Reprod Immunol. 2015;108:33–41.
Blois SM, Ilarregui JM, Tometten M, Garcia M, Orsal AS, Cordo-Russo R, et al. A pivotal role for galectin-1 in fetomaternal tolerance. Nat Med. 2007;13(12):1450–7.
van der Leij J, van den Berg A, Blokzijl T, Harms G, van Goor H, Zwiers P, et al. Dimeric galectin-1 induces IL-10 production in T-lymphocytes: an important tool in the regulation of the immune response. J Pathol. 2004;204(5):511–8.
Kopcow HD, Rosetti F, Leung Y, Allan DS, Kutok JL, Strominger JL. T cell apoptosis at the maternal-fetal interface in early human pregnancy, involvement of galectin-1. Proc Natl Acad Sci U S A. 2008;105(47):18472–7.
Rostoker R, Yaseen H, Schif-Zuck S, Lichtenstein RG, Rabinovich GA, Ariel A. Galectin-1 induces 12/15-lipoxygenase expression in murine macrophages and favors their conversion toward a pro-resolving phenotype. Prostaglandins Other Lipid Mediat. 2013;107:85–94.
Jeschke U, Mayr D, Schiessl B, Mylonas I, Schulze S, Kuhn C, et al. Expression of galectin-1, −3 (gal-1, gal-3) and the Thomsen-Friedenreich (TF) antigen in normal, IUGR, preeclamptic and HELLP placentas. Placenta. 2007;28(11–12):1165–73.
Than NG, Erez O, Wildman DE, Tarca AL, Edwin SS, Abbas A, et al. Severe preeclampsia is characterized by increased placental expression of galectin-1. J Matern Fetal Neonatal Med. 2008;21(7):429–42.
Freitag N, Tirado-González I, Barrientos G, Herse F, Thijssen VL, Weedon-Fekjær SM, et al. Interfering with Gal-1-mediated angiogenesis contributes to the pathogenesis of preeclampsia. Proc Natl Acad Sci U S A. 2013;110(28):11451–6.
Blois SM, Dechend R, Barrientos G, Staff AC. A potential pathophysiological role for galectins and the renin-angiotensin system in preeclampsia. Cell Mol Life Sci. 2015;72(1):39–50.
Molvarec A, Blois SM, Stenczer B, Toldi G, Tirado-Gonzalez I, Ito M, et al. Peripheral blood galectin-1-expressing T and natural killer cells in normal pregnancy and preeclampsia. Clin Immunol. 2011;139(1):48–56.
Than NG, Pick E, Bellyei S, Szigeti A, Burger O, Berente Z, et al. Functional analyses of placental protein 13/galectin-13. Eur J Biochem. 2004;271(6):1065–78.
Orendi K, Gauster M, Moser G, Meiri H, Huppertz B. The choriocarcinoma cell line BeWo: syncytial fusion and expression of syncytium-specific proteins. Reproduction. 2010;140(5):759–66.
Than NG, Romero R, Xu Y, Erez O, Xu Z, Bhatti G, et al. Evolutionary origins of the placental expression of chromosome 19 cluster galectins and their complex dysregulation in preeclampsia. Placenta. 2014;35(11):855–65.
Than NG, Romero R, Goodman M, Weckle A, Xing J, Dong Z, et al. A primate subfamily of galectins expressed at the maternal-fetal interface that promote immune cell death. Proc Natl Acad Sci U S A. 2009;106(24):9731–6.
Kliman HJ, Sammar M, Grimpel YI, Lynch SK, Milano KM, Pick E, et al. Placental protein 13 and decidual zones of necrosis: an immunologic diversion that may be linked to preeclampsia. Reprod Sci. 2012;19(1):16–30.
Than NG, Abdul Rahman O, Magenheim R, Nagy B, Fule T, Hargitai B, et al. Placental protein 13 (galectin-13) has decreased placental expression but increased shedding and maternal serum concentrations in patients presenting with preterm pre-eclampsia and HELLP syndrome. Virchows Arch. 2008;453(4):387–400.
Huppertz B, Sammar M, Chefetz I, Neumaier-Wagner P, Bartz C, Meiri H. Longitudinal determination of serum placental protein 13 during development of preeclampsia. Fetal Diagn Ther. 2008;24(3):230–6.
Gonen R, Shahar R, Grimpel YI, Chefetz I, Sammar M, Meiri H, et al. Placental protein 13 as an early marker for pre-eclampsia: a prospective longitudinal study. BJOG. 2008;115(12):1465–72.
Sekizawa A, Purwosunu Y, Yoshimura S, Nakamura M, Shimizu H, Okai T, et al. PP13 mRNA expression in trophoblasts from preeclamptic placentas. Reprod Sci. 2009;16(4):408–13.
Shimizu H, Sekizawa A, Purwosunu Y, Nakamura M, Farina A, Rizzo N, et al. PP13 mRNA expression in the cellular component of maternal blood as a marker for preeclampsia. Prenat Diagn. 2009;29(13):1231–6.
Farina A, Zucchini C, Sekizawa A, Purwosunu Y, de Sanctis P, Santarsiero G, et al. Performance of messenger RNAs circulating in maternal blood in the prediction of preeclampsia at 10–14 weeks. Am J Obstet Gynecol. 2010;203(6):575.e1–7.
Gebhardt S, Bruiners N, Hillermann R. A novel exonic variant (221delT) in the LGALS13 gene encoding placental protein 13 (PP13) is associated with preterm labour in a low risk population. J Reprod Immunol. 2009;82(2):166–73.
Than NG, Balogh A, Romero R, Kárpáti E, Erez O, Szilágyi A, et al. Placental protein 13 (PP13)—a placental Immunoregulatory galectin protecting pregnancy. Front Immunol. 2014;5:348.
Huppertz B, Meiri H, Gizurarson S, Osol G, Sammar M. Placental protein 13 (PP13): a new biological target shifting individualized risk assessment to personalized drug design combating pre-eclampsia. Hum Reprod Update. 2013;19(4):391–405.
Sammar M, Nisemblat S, Fleischfarb Z, Golan A, Sadan O, Meiri H, et al. Placenta-bound and body fluid PP13 and its mRNA in normal pregnancy compared to preeclampsia, HELLP and preterm delivery. Placenta. 2011;32(Suppl):S30–6.
Zabel BA, Kwitniewski M, Banas M, Zabieglo K, Murzyn K, Cichy J. Chemerin regulation and role in host defense. Am J Clin Exp Immunol. 2014;3(1):1–19.
Carlino C, Trotta E, Stabile H, Morrone S, Bulla R, Soriani A, et al. Chemerin regulates NK cell accumulation and endothelial cell morphogenesis in the decidua during early pregnancy. J Clin Endocrinol Metab. 2012;97(10):3603–12.
Tessier DR, Yockell-Lelievre J, Gruslin A. Uterine spiral artery remodeling: the role of uterine natural killer cells and extravillous trophoblasts in normal and high-risk human pregnancies. Am J Reprod Immunol. 2015;74(1):1–11.
Kaur J, Adya R, Tan BK, Chen J, Randeva HS. Identification of chemerin receptor (ChemR23) in human endothelial cells: chemerin-induced endothelial angiogenesis. Biochem Biophys Res Commun. 2010;391(4):1762–8.
Mariani F, Roncucci L. Chemerin/chemR23 axis in inflammation onset and resolution. Inflamm Res. 2015;64(2):85–95.
Moretta A, Marcenaro E, Parolini S, Ferlazzo G, Moretta L. NK cells at the interface between innate and adaptive immunity. Cell Death Differ. 2008;15(2):226–33.
Hespel C, Moser M. Role of inflammatory dendritic cells in innate and adaptive immunity. Eur J Immunol. 2012;42(10):2535–43.
Zabel BA, Allen SJ, Kulig P, Allen JA, Cichy J, Handel TM, et al. Chemerin activation by serine proteases of the coagulation, fibrinolytic, and inflammatory cascades. J Biol Chem. 2005;280(41):34661–6.
Garces MF, Sanchez E, Ruiz-Parra AI, Rubio-Romero JA, Angel-Muller E, Suarez MA, et al. Serum chemerin levels during normal human pregnancy. Peptides. 2013;42:138–43.
Kasher-Meron M, Mazaki-Tovi S, Barhod E, Hemi R, Haas J, Gat I, et al. Chemerin concentrations in maternal and fetal compartments: implications for metabolic adaptations to normal human pregnancy. J Perinat Med. 2014;42(3):371–8.
Wang L, Yang T, Ding Y, Zhong Y, Yu L, Peng M. Chemerin plays a protective role by regulating human umbilical vein endothelial cell-induced nitric oxide signaling in preeclampsia. Endocrine. 2015;48(1):299–308.
Duan DM, Niu JM, Lei Q, Lin XH, Chen X. Serum levels of the adipokine chemerin in preeclampsia. J Perinat Med. 2012;40(2):121–7.
Stepan H, Philipp A, Roth I, Kralisch S, Jank A, Schaarschmidt W, et al. Serum levels of the adipokine chemerin are increased in preeclampsia during and 6 months after pregnancy. Regul Pept. 2011;168(1–3):69–72.
Xu QL, Zhu M, Jin Y, Wang N, Xu HX, Quan LM, et al. The predictive value of the first-trimester maternal serum chemerin level for pre-eclampsia. Peptides. 2014;62:150–4.
Serhan CN. Pro-resolving lipid mediators are leads for resolution physiology. Nature. 2014;510(7503):92–101.
Claria J, Serhan CN. Aspirin triggers previously undescribed bioactive eicosanoids by human endothelial cell-leukocyte interactions. Proc Natl Acad Sci U S A. 1995;92(21):9475–9.
Serhan CN. Lipoxins and aspirin-triggered 15-epi-lipoxins are the first lipid mediators of endogenous anti-inflammation and resolution. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):141–62.
Fierro IM. Angiogenesis and lipoxins. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):271–5.
Choi G, Hwang SW. Modulation of the activities of neuronal ion channels by fatty acid-derived pro-resolvents. Front Physiol. 2016;7:523.
Levy BD, Serhan CN. Exploring new approaches to the treatment of asthma: potential roles for lipoxins and aspirin-triggered lipid mediators. Drugs Today (Barc). 2003;39(5):373–84.
Lee TH. Lipoxin A4: a novel anti-inflammatory molecule? Thorax. 1995;50(2):111–2.
Gavins FN, Sawmynaden P, Chatterjee BE, Perretti M. A twist in anti-inflammation: annexin 1 acts via the lipoxin A4 receptor. Prostaglandins Leukot Essent Fatty Acids. 2005;73(3–4):211–9.
Wang J, Huang Y, Zhou J, Liu X. Effect of lipoxin A4 on IL-1β production of monocytes and its possible mechanism in severe preeclampsia. J Huazhong Univ Sci Technolog Med Sci. 2010;30(6):767–70.
Gil-Villa AM, Norling LV, Serhan CN, Cordero D, Rojas M, Cadavid A. Aspirin triggered-lipoxin A4 reduces the adhesion of human polymorphonuclear neutrophils to endothelial cells initiated by preeclamptic plasma. Prostaglandins Leukot Essent Fatty Acids. 2012;87(4–5):127–34.
Lin F, Zeng P, Xu Z, Ye D, Yu X, Wang N, et al. Treatment of lipoxin a(4) and its analogue on low-dose endotoxin induced preeclampsia in rat and possible mechanisms. Reprod Toxicol. 2012;34(4):677–85.
Huang LL, Su S, Awale R, Zhang XY, Zhong LL, Tang H. Expression of anti-inflammatory mediator lipoxin A4 and inflammation responsive transcriptive factors NF-kappa B in patients with preeclampsia. Clin Exp Obstet Gynecol. 2014;41(5):561–6.
Perucci LO, Santos PC, Ribeiro LS, Souza DG, Gomes KB, Dusse LM, et al. Lipoxin A4 is increased in the plasma of preeclamptic women. Am J Hypertens. 2016;29(10):1179–85.
Cooper CE, Brown GC. The inhibition of mitochondrial cytochrome oxidase by the gases carbon monoxide, nitric oxide, hydrogen cyanide and hydrogen sulfide: chemical mechanism and physiological significance. J Bioenerg Biomembr. 2008;40(5):533–9.
Wang R. Two’s company, three’s a crowd: can H2S be the third endogenous gaseous transmitter? FASEB J. 2002;16(13):1792–8.
Kajimura M, Fukuda R, Bateman RM, Yamamoto T, Suematsu M. Interactions of multiple gas-transducing systems: hallmarks and uncertainties of CO, NO, and H2S gas biology. Antioxid Redox Signal. 2010;13(2):157–92.
Lyall F. Development of the utero-placental circulation: the role of carbon monoxide and nitric oxide in trophoblast invasion and spiral artery transformation. Microsc Res Tech. 2003;60(4):402–11.
Li H, Horke S, Förstermann U. Vascular oxidative stress, nitric oxide and atherosclerosis. Atherosclerosis. 2014;237(1):208–19.
Nagy G, Koncz A, Telarico T, Fernandez D, Ersek B, Buzás E, et al. Central role of nitric oxide in the pathogenesis of rheumatoid arthritis and systemic lupus erythematosus. Arthritis Res Ther. 2010;12(3):210.
Ignarro LJ, Buga GM, Wood KS, Byrns RE, Chaudhuri G. Endothelium-derived relaxing factor produced and released from artery and vein is nitric oxide. Proc Natl Acad Sci U S A. 1987;84(24):9265–9.
Sogo N, Magid KS, Shaw CA, Webb DJ, Megson IL. Inhibition of human platelet aggregation by nitric oxide donor drugs: relative contribution of cGMP-independent mechanisms. Biochem Biophys Res Commun. 2000;279(2):412–9.
Ward C, Wong TH, Murray J, Rahman I, Haslett C, Chilvers ER, et al. Induction of human neutrophil apoptosis by nitric oxide donors: evidence for a caspase-dependent, cyclic-GMP-independent, mechanism. Biochem Pharmacol. 2000;59(3):305–14.
Lo Faro ML, Fox B, Whatmore JL, Winyard PG, Whiteman M. Hydrogen sulfide and nitric oxide interactions in inflammation. Nitric Oxide. 2014;41:38–47.
Laroux FS, Lefer DJ, Kawachi S, Scalia R, Cockrell AS, Gray L, et al. Role of nitric oxide in the regulation of acute and chronic inflammation. Antioxid Redox Signal. 2000;2(3):391–6.
Guzik TJ, Korbut R, Adamek-Guzik T. Nitric oxide and superoxide in inflammation and immune regulation. J Physiol Pharmacol. 2003;54(4):469–87.
Shaw CA, Taylor EL, Fox S, Megson IL, Rossi AG. Differential susceptibility to nitric oxide-evoked apoptosis in human inflammatory cells. Free Radic Biol Med. 2011;50(1):93–101.
Huang LT, Hsieh CS, Chang KA, Tain YL. Roles of nitric oxide and asymmetric dimethylarginine in pregnancy and fetal programming. Int J Mol Sci. 2012;13(11):14606–22.
Morris NH, Sooranna SR, Learmont JG, Poston L, Ramsey B, Pearson JD, et al. Nitric oxide synthase activities in placental tissue from normotensive, pre-eclamptic and growth retarded pregnancies. Br J Obstet Gynaecol. 1995;102(9):711–4.
Böger RH, Diemert A, Schwedhelm E, Lüneburg N, Maas R, Hecher K. The role of nitric oxide synthase inhibition by asymmetric dimethylarginine in the pathophysiology of preeclampsia. Gynecol Obstet Investig. 2010;69(1):1–13.
López-Jaramillo P, Arenas WD, García RG, Rincon MY, López M. The role of the L-arginine-nitric oxide pathway in preeclampsia. Ther Adv Cardiovasc Dis. 2008;2(4):261–75.
Bian Z, Shixia C, Duan T. First-trimester maternal serum levels of sFLT1, PGF and ADMA predict preeclampsia. PLoS One. 2015;10(4):e0124684.
Alpoim PN, Godoi LC, Freitas LG, Gomes KB, Dusse LM. Assessment of L-arginine asymmetric 1 dimethyl (ADMA) in early-onset and late-onset (severe) preeclampsia. Nitric Oxide. 2013;33:81–2.
Alpoim PN, Gomes KB, Pinheiro MB, Godoi LC, Jardim LL, Muniz LG, et al. Polymorphisms in endothelial nitric oxide synthase gene in early and late severe preeclampsia. Nitric Oxide. 2014;42:19–23.
Doridot L, Passet B, Mehats C, Rigourd V, Barbaux S, Ducat A, et al. Preeclampsia-like symptoms induced in mice by fetoplacental expression of STOX1 are reversed by aspirin treatment. Hypertension. 2013;61(3):662–8.
van Dijk M, Mulders J, Poutsma A, Konst AA, Lachmeijer AM, Dekker GA, et al. Maternal segregation of the Dutch preeclampsia locus at 10q22 with a new member of the winged helix gene family. Nat Genet. 2005;37(5):514–9.
Doridot L, Chatre L, Ducat A, Vilotte JL, Lombes A, Mehats C, et al. Nitroso-redox balance and mitochondrial homeostasis are regulated by STOX1, a pre-eclampsia-associated gene. Antioxid Redox Signal. 2014;21(6):819–34.
van Dijk M, van Bezu J, van Abel D, Dunk C, Blankenstein MA, Oudejans CB, et al. The STOX1 genotype associated with pre-eclampsia leads to a reduction of trophoblast invasion by alpha-T-catenin upregulation. Hum Mol Genet. 2010;19(13):2658–67.
Nanaev A, Chwalisz K, Frank HG, Kohnen G, Hegele-Hartung C, Kaufmann P. Physiological dilation of uteroplacental arteries in the guinea pig depends on nitric oxide synthase activity of extravillous trophoblast. Cell Tissue Res. 1995;282(3):407–21.
Holwerda KM, Faas MM, van Goor H, Lely AT. Gasotransmitters: a solution for the therapeutic dilemma in preeclampsia? Hypertension. 2013;62(4):653–9.
Meher S, Duley L. Nitric oxide for preventing pre-eclampsia and its complications. Cochrane Database Syst Rev. 2007;2:CD006490.
Olas B. Hydrogen sulfide in signaling pathways. Clin Chim Acta. 2015;439:212–8.
Kida M, Sugiyama T, Yoshimoto T, Ogawa Y. Hydrogen sulfide increases nitric oxide production with calcium-dependent activation of endothelial nitric oxide synthase in endothelial cells. Eur J Pharm Sci. 2013;48(1–2):211–5.
Szabó C. Hydrogen sulphide and its therapeutic potential. Nat Rev Drug Discov. 2007;6(11):917–35.
Collin M, Anuar FB, Murch O, Bhatia M, Moore PK, Thiemermann C. Inhibition of endogenous hydrogen sulfide formation reduces the organ injury caused by endotoxemia. Br J Pharmacol. 2005;146(4):498–505.
Zhang H, Zhi L, Moochhala S, Moore PK, Bhatia M. Hydrogen sulfide acts as an inflammatory mediator in cecal ligation and puncture-induced sepsis in mice by upregulating the production of cytokines and chemokines via NF-kappaB. Am J Physiol Lung Cell Mol Physiol. 2007;292(4):L960–71.
Wallace JL, Vong L, McKnight W, Dicay M, Martin GR. Endogenous and exogenous hydrogen sulfide promotes resolution of colitis in rats. Gastroenterology. 2009;137(2):569–78. 78.e1
Mariggio MA, Minunno V, Riccardi S, Santacroce R, De Rinaldis P, Fumarulo R. Sulfide enhancement of PMN apoptosis. Immunopharmacol Immunotoxicol. 1998;20(3):399–408.
Miao L, Shen X, Whiteman M, Xin H, Shen Y, Xin X, et al. Hydrogen sulfide mitigates myocardial infarction via promotion of mitochondrial biogenesis-dependent M2 polarization of macrophages. Antioxid Redox Signal. 2016;25(5):268–81.
Dufton N, Natividad J, Verdu EF, Wallace JL. Hydrogen sulfide and resolution of acute inflammation: a comparative study utilizing a novel fluorescent probe. Sci Rep. 2012;2:499.
Brancaleone V, Mitidieri E, Flower RJ, Cirino G, Perretti M. Annexin A1 mediates hydrogen sulfide properties in the control of inflammation. J Pharmacol Exp Ther. 2014;351(1):96–104.
Papapetropoulos A, Pyriochou A, Altaany Z, Yang G, Marazioti A, Zhou Z, et al. Hydrogen sulfide is an endogenous stimulator of angiogenesis. Proc Natl Acad Sci U S A. 2009;106(51):21972–7.
Patel P, Vatish M, Heptinstall J, Wang R, Carson RJ. The endogenous production of hydrogen sulphide in intrauterine tissues. Reprod Biol Endocrinol. 2009;7:10.
Wang K, Ahmad S, Cai M, Rennie J, Fujisawa T, Crispi F, et al. Dysregulation of hydrogen sulfide producing enzyme cystathionine γ-lyase contributes to maternal hypertension and placental abnormalities in preeclampsia. Circulation. 2013;127(25):2514–22.
Cindrova-Davies T, Herrera EA, Niu Y, Kingdom J, Giussani DA, Burton GJ. Reduced cystathionine γ-lyase and increased miR-21 expression are associated with increased vascular resistance in growth-restricted pregnancies: hydrogen sulfide as a placental vasodilator. Am J Pathol. 2013;182(4):1448–58.
Holwerda KM, Bos EM, Rajakumar A, Ris-Stalpers C, van Pampus MG, Timmer A, et al. Hydrogen sulfide producing enzymes in pregnancy and preeclampsia. Placenta. 2012;33(6):518–21.
Roes EM, Raijmakers MT, Boo TM, Zusterzeel PL, Merkus HM, Peters WH, et al. Oral N-acetylcysteine administration does not stabilise the process of established severe preeclampsia. Eur J Obstet Gynecol Reprod Biol. 2006;127(1):61–7.
Ryter SW, Choi AM. Heme oxygenase-1/carbon monoxide: from metabolism to molecular therapy. Am J Respir Cell Mol Biol. 2009;41(3):251–60.
Wu L, Wang R. Carbon monoxide: endogenous production, physiological functions, and pharmacological applications. Pharmacol Rev. 2005;57(4):585–630.
Bilban M, Haschemi A, Wegiel B, Chin BY, Wagner O, Otterbein LE. Heme oxygenase and carbon monoxide initiate homeostatic signaling. J Mol Med (Berl). 2008;86(3):267–79.
Peers C, Boyle JP, Scragg JL, Dallas ML, Al-Owais MM, Hettiarachichi NT, et al. Diverse mechanisms underlying the regulation of ion channels by carbon monoxide. Br J Pharmacol. 2015;172(6):1546–56.
Urquhart P, Rosignoli G, Cooper D, Motterlini R, Perretti M. Carbon monoxide-releasing molecules modulate leukocyte-endothelial interactions under flow. J Pharmacol Exp Ther. 2007;321(2):656–62.
Morse D, Pischke SE, Zhou Z, Davis RJ, Flavell RA, Loop T, et al. Suppression of inflammatory cytokine production by carbon monoxide involves the JNK pathway and AP-1. J Biol Chem. 2003;278(39):36993–8.
Otterbein LE, May A, Chin BY. Carbon monoxide increases macrophage bacterial clearance through toll-like receptor (TLR)4 expression. Cell Mol Biol (Noisy-le-grand). 2005;51(5):433–40.
Chiang N, Shinohara M, Dalli J, Mirakaj V, Kibi M, Choi AM, et al. Inhaled carbon monoxide accelerates resolution of inflammation via unique proresolving mediator-heme oxygenase-1 circuits. J Immunol. 2013;190(12):6378–88.
Lyall F, Barber A, Myatt L, Bulmer JN, Robson SC. Hemeoxygenase expression in human placenta and placental bed implies a role in regulation of trophoblast invasion and placental function. FASEB J. 2000;14(1):208–19.
Bainbridge SA, Smith GN. HO in pregnancy. Free Radic Biol Med. 2005;38(8):979–88.
McCaig D, Lyall F. Inhibitors of heme oxygenase reduce invasion of human primary cytotrophoblast cells in vitro. Placenta. 2009;30(6):536–8.
Sollwedel A, Bertoja AZ, Zenclussen ML, Gerlof K, Lisewski U, Wafula P, et al. Protection from abortion by heme oxygenase-1 up-regulation is associated with increased levels of bag-1 and neuropilin-1 at the fetal-maternal interface. J Immunol. 2005;175(8):4875–85.
Baum M, Schiff E, Kreiser D, Dennery PA, Stevenson DK, Rosenthal T, et al. End-tidal carbon monoxide measurements in women with pregnancy-induced hypertension and preeclampsia. Am J Obstet Gynecol. 2000;183(4):900–3.
Yusuf K, Kamaluddeen M, Wilson RD, Akierman A. Carboxyhemoglobin levels in umbilical cord blood of women with pre-eclampsia and intrauterine growth restriction. J Perinat Med. 2012;40(6):619–24.
Zhai D, Guo Y, Smith G, Krewski D, Walker M, Wen SW. Maternal exposure to moderate ambient carbon monoxide is associated with decreased risk of preeclampsia. Am J Obstet Gynecol. 2012;207(1):57.e1–9.
Wikström AK, Stephansson O, Cnattingius S. Tobacco use during pregnancy and preeclampsia risk: effects of cigarette smoking and snuff. Hypertension. 2010;55(5):1254–9.
Maebayashi Asanuma A, Yamamoto T, Azuma H, Kato E, Yamamoto N, Murase T, et al. Expression of placenta growth factor, soluble fms-like tyrosine kinase-1, metal-responsive transcription factor-1, heme oxygenase 1 and hypoxia inducible factor-1α mRNAs in pre-eclampsia placenta and the effect of pre-eclampsia sera on their expression of choriocarcinoma cells. J Obstet Gynaecol Res. 2014;40(10):2095–103.
Zenclussen AC, Lim E, Knoeller S, Knackstedt M, Hertwig K, Hagen E, et al. Heme oxygenases in pregnancy II: HO-2 is downregulated in human pathologic pregnancies. Am J Reprod Immunol. 2003;50(1):66–76.
Barber A, Robson SC, Myatt L, Bulmer JN, Lyall F. Heme oxygenase expression in human placenta and placental bed: reduced expression of placenta endothelial HO-2 in preeclampsia and fetal growth restriction. FASEB J. 2001;15(7):1158–68.
Eide IP, Isaksen CV, Salvesen KA, Langaas M, Schønberg SA, Austgulen R. Decidual expression and maternal serum levels of heme oxygenase 1 are increased in pre-eclampsia. Acta Obstet Gynecol Scand. 2008;87(3):272–9.
Tong S, Kaitu’u-Lino TJ, Onda K, Beard S, Hastie R, Binder NK, et al. Heme oxygenase-1 is not decreased in preeclamptic placenta and does not negatively regulate placental soluble fms-like tyrosine kinase-1 or soluble endoglin secretion. Hypertension. 2015;66(5):1073–81.
Brownfoot FC, Tong S, Hannan NJ, Binder NK, Walker SP, Cannon P, et al. Effects of pravastatin on human placenta, endothelium, and women with severe preeclampsia. Hypertension. 2015;66(3):687–97. discussion 445
Ramma W, Ahmed A. Therapeutic potential of statins and the induction of heme oxygenase-1 in preeclampsia. J Reprod Immunol. 2014;101-102:153–60.
McCarthy FP, Drewlo S, Kingdom J, Johns EJ, Walsh SK, Kenny LC. Peroxisome proliferator-activated receptor-γ as a potential therapeutic target in the treatment of preeclampsia. Hypertension. 2011;58(2):280–6.
Venditti CC, Casselman R, Young I, Karumanchi SA, Smith GN. Carbon monoxide prevents hypertension and proteinuria in an adenovirus sFlt-1 preeclampsia-like mouse model. PLoS One. 2014;9(9):e106502.
Picciotto MR, Higley MJ, Mineur YS. Acetylcholine as a neuromodulator: cholinergic signaling shapes nervous system function and behavior. Neuron. 2012;76(1):116–29.
Prado MA, Reis RA, Prado VF, de Mello MC, Gomez MV, de Mello FG. Regulation of acetylcholine synthesis and storage. Neurochem Int. 2002;41(5):291–9.
Kellogg Jr DL, Zhao JL, Coey U, Green JV. Acetylcholine-induced vasodilation is mediated by nitric oxide and prostaglandins in human skin. J Appl Physiol (1985). 2005;98(2):629–32.
Laurent P, Safar ME, Meaune S, Blacher J. Influence of L-nitro-arginine methyl ester, acetylcholine, and adenosine on mean blood pressure, pulse pressure, and pulse pressure amplification in rats. J Cardiovasc Pharmacol. 2003;41(2):210–8.
Andersson U, Tracey KJ. Reflex principles of immunological homeostasis. Annu Rev Immunol. 2012;30:313–35.
Andersson U, Tracey KJ. Neural reflexes in inflammation and immunity. J Exp Med. 2012;209(6):1057–68.
Sundman E, Olofsson PS. Neural control of the immune system. Adv Physiol Educ. 2014;38(2):135–9.
Báez-Pagán CA, Delgado-Vélez M, Lasalde-Dominicci JA. Activation of the macrophage α7 nicotinic acetylcholine receptor and control of inflammation. J NeuroImmune Pharmacol. 2015;10(3):468–76.
Tsoyi K, Jang HJ, Kim JW, Chang HK, Lee YS, Pae HO, et al. Stimulation of alpha7 nicotinic acetylcholine receptor by nicotine attenuates inflammatory response in macrophages and improves survival in experimental model of sepsis through heme oxygenase-1 induction. Antioxid Redox Signal. 2011;14(11):2057–70.
Maldifassi MC, Atienza G, Arnalich F, López-Collazo E, Cedillo JL, Martín-Sánchez C, et al. A new IRAK-M-mediated mechanism implicated in the anti-inflammatory effect of nicotine via α7 nicotinic receptors in human macrophages. PLoS One. 2014;9(9):e108397.
Huston JM, Rosas-Ballina M, Xue X, Dowling O, Ochani K, Ochani M, et al. Cholinergic neural signals to the spleen down-regulate leukocyte trafficking via CD11b. J Immunol. 2009;183(1):552–9.
Mariggiò MA, Guida L, Laforgia A, Santacroce R, Curci E, Montemurro P, et al. Nicotine effects on polymorphonuclear cell apoptosis and lipopolysaccharide-induced monocyte functions. A possible role in periodontal disease? J Periodontal Res. 2001;36(1):32–9.
van der Zanden EP, Snoek SA, Heinsbroek SE, Stanisor OI, Verseijden C, Boeckxstaens GE, et al. Vagus nerve activity augments intestinal macrophage phagocytosis via nicotinic acetylcholine receptor alpha4beta2. Gastroenterology. 2009;137(3):1029–39. 39.e1-4
Lee RH, Vazquez G. Evidence for a prosurvival role of alpha-7 nicotinic acetylcholine receptor in alternatively (M2)-activated macrophages. Physiol Rep. 2013;1(7):e00189.
Tracey KJ. Physiology and immunology of the cholinergic antiinflammatory pathway. J Clin Invest. 2007;117(2):289–96.
Mirakaj V, Dalli J, Granja T, Rosenberger P, Serhan CN. Vagus nerve controls resolution and pro-resolving mediators of inflammation. J Exp Med. 2014;211(6):1037–48.
Yang CC, Chao TC, Kuo TB, Yin CS, Chen HI. Preeclamptic pregnancy is associated with increased sympathetic and decreased parasympathetic control of HR. Am J Physiol Heart Circ Physiol. 2000;278(4):H1269–73.
Dowling O, Rochelson B, Way K, Al-Abed Y, Metz CN. Nicotine inhibits cytokine production by placenta cells via NFkappaB: potential role in pregnancy-induced hypertension. Mol Med. 2007;13(11–12):576–83.
Liu Y, Yang J, Bao J, Li X, Ye A, Zhang G, et al. Activation of the cholinergic anti-inflammatory pathway by nicotine ameliorates lipopolysaccharide-induced preeclampsia-like symptoms in pregnant rats. Placenta. 2017;49:23–32.
Mimura K, Tomimatsu T, Sharentuya N, Tskitishvili E, Kinugasa-Taniguchi Y, Kanagawa T, et al. Nicotine restores endothelial dysfunction caused by excess sFlt1 and sEng in an in vitro model of preeclamptic vascular endothelium: a possible therapeutic role of nicotinic acetylcholine receptor (nAChR) agonists for preeclampsia. Am J Obstet Gynecol. 2010;202(5):464 e1–6.
Machaalani R, Ghazavi E, David RV, Hinton T, Makris A, Hennessy A. Nicotinic acetylcholine receptors (nAChR) are increased in the pre-eclamptic placenta. Hypertens Pregnancy. 2015;34(2):227–40.
Kwon JY, Kim YH, Kim SH, Kang MH, Maeng YS, Lee KY, et al. Difference in the expression of alpha 7 nicotinic receptors in the placenta in normal versus severe preeclampsia pregnancies. Eur J Obstet Gynecol Reprod Biol. 2007;132(1):35–9.
Bradford D, Cole SJ, Cooper HM. Netrin-1: diversity in development. Int J Biochem Cell Biol. 2009;41(3):487–93.
Ly NP, Komatsuzaki K, Fraser IP, Tseng AA, Prodhan P, Moore KJ, et al. Netrin-1 inhibits leukocyte migration in vitro and in vivo. Proc Natl Acad Sci U S A. 2005;102(41):14729–34.
Aherne CM, Collins CB, Masterson JC, Tizzano M, Boyle TA, Westrich JA, et al. Neuronal guidance molecule netrin-1 attenuates inflammatory cell trafficking during acute experimental colitis. Gut. 2012;61(5):695–705.
Ranganathan PV, Jayakumar C, Mohamed R, Dong Z, Ramesh G. Netrin-1 regulates the inflammatory response of neutrophils and macrophages, and suppresses ischemic acute kidney injury by inhibiting COX-2-mediated PGE2 production. Kidney Int. 2013;83(6):1087–98.
Tadagavadi RK, Wang W, Ramesh G. Netrin-1 regulates Th1/Th2/Th17 cytokine production and inflammation through UNC5B receptor and protects kidney against ischemia-reperfusion injury. J Immunol. 2010;185(6):3750–8.
Ranganathan PV, Jayakumar C, Ramesh G. Netrin-1-treated macrophages protect the kidney against ischemia-reperfusion injury and suppress inflammation by inducing M2 polarization. Am J Physiol Renal Physiol. 2013;304(7):F948–57.
Schlegel M, Köhler D, Körner A, Granja T, Straub A, Giera M, et al. The neuroimmune guidance cue netrin-1 controls resolution programs and promotes liver regeneration. Hepatology. 2015.
Carney EF. Diabetic nephropathy: netrin-1 expression in proximal tubular epithelial cells protects against kidney inflammation and injury. Nat Rev Nephrol. 2012;8(12):681.
Mirakaj V, Gatidou D, Pötzsch C, König K, Rosenberger P. Netrin-1 signaling dampens inflammatory peritonitis. J Immunol. 2011;186(1):549–55.
Podjaski C, Alvarez JI, Bourbonniere L, Larouche S, Terouz S, Bin JM, et al. Netrin 1 regulates blood-brain barrier function and neuroinflammation. Brain. 2015;138(Pt 6):1598–612.
Yang Y, Zou L, Xu KS. Expression of netrin-1 in placenta from patients with pre-eclampsia and the relation to placental angiogenesis. Zhonghua Fu Chan Ke Za Zhi. 2006;41(9):597–600.
López-Novoa JM, Bernabeu C. The physiological role of endoglin in the cardiovascular system. Am J Physiol Heart Circ Physiol. 2010;299(4):H959–74.
Powers JC, Odake S, Oleksyszyn J, Hori H, Ueda T, Boduszek B, et al. Proteases—structures, mechanism and inhibitors. Agents Actions Suppl. 1993;42:3–18.
Mancek-Keber M. Inflammation-mediating proteases: structure, function in (patho) physiology and inhibition. Protein Pept Lett. 2014;21(12):1209–29.
Greene CM, McElvaney NG. Proteases and antiproteases in chronic neutrophilic lung disease—relevance to drug discovery. Br J Pharmacol. 2009;158(4):1048–58.
Apte SS, Parks WC. Metalloproteinases: a parade of functions in matrix biology and an outlook for the future. Matrix Biol. 2015;44-46:1–6.
Cohen M, Meisser A, Bischof P. Metalloproteinases and human placental invasiveness. Placenta. 2006;27(8):783–93.
Murphy G. Tissue inhibitors of metalloproteinases. Genome Biol. 2011;12(11):233.
Khokha R, Murthy A, Weiss A. Metalloproteinases and their natural inhibitors in inflammation and immunity. Nat Rev Immunol. 2013;13(9):649–65.
Nissinen L, Kähäri VM. Matrix metalloproteinases in inflammation. Biochim Biophys Acta. 2014;1840(8):2571–80.
Black RA, Doedens JR, Mahimkar R, Johnson R, Guo L, Wallace A, et al. Substrate specificity and inducibility of TACE (tumour necrosis factor alpha-converting enzyme) revisited: the ala-Val preference, and induced intrinsic activity. Biochem Soc Symp. 2003;70:39–52.
Tang J, Zarbock A, Gomez I, Wilson CL, Lefort CT, Stadtmann A, et al. Adam17-dependent shedding limits early neutrophil influx but does not alter early monocyte recruitment to inflammatory sites. Blood. 2011;118(3):786–94.
Wang Y, Robertson JD, Walcheck B. Different signaling pathways stimulate a disintegrin and metalloprotease-17 (ADAM17) in neutrophils during apoptosis and activation. J Biol Chem. 2011;286(45):38980–8.
Lakatos G, Hritz I, Varga MZ, Juhász M, Miheller P, Cierny G, et al. The impact of matrix metalloproteinases and their tissue inhibitors in inflammatory bowel diseases. Dig Dis. 2012;30(3):289–95.
Ma R, Gu Y, Groome LJ, Wang Y. ADAM17 regulates TNFα production by placental trophoblasts. Placenta. 2011;32(12):975–80.
Ma R, Gu B, Gu Y, Groome LJ, Wang Y. Down-regulation of TIMP3 leads to increase in TACE expression and TNFα production by placental trophoblast cells. Am J Reprod Immunol. 2014;71(5):427–33.
Perucci LO, Gomes KB, Freitas LG, Godoi LC, Alpoim PN, Pinheiro MB, et al. Soluble endoglin, transforming growth factor-Beta 1 and soluble tumor necrosis factor alpha receptors in different clinical manifestations of preeclampsia. PLoS One. 2014;9(5):e97632.
Pinheiro MB, Martins-Filho OA, Mota AP, Alpoim PN, Godoi LC, Silveira AC, et al. Severe preeclampsia goes along with a cytokine network disturbance towards a systemic inflammatory state. Cytokine. 2013;62(1):165–73.
Vince GS, Starkey PM, Austgulen R, Kwiatkowski D, Redman CW. Interleukin-6, tumour necrosis factor and soluble tumour necrosis factor receptors in women with pre-eclampsia. Br J Obstet Gynaecol. 1995;102(1):20–5.
Palei AC, Granger JP, Tanus-Santos JE. Matrix metalloproteinases as drug targets in preeclampsia. Curr Drug Targets. 2013;14(3):325–34.
Prieto P, Cuenca J, Traves PG, Fernandez-Velasco M, Martin-Sanz P, Bosca L. Lipoxin A4 impairment of apoptotic signaling in macrophages: implication of the PI3K/Akt and the ERK/Nrf-2 defense pathways. Cell Death Differ. 2010;17(7):1179–88.
El Kebir D, Jozsef L, Pan W, Wang L, Petasis NA, Serhan CN, et al. 15-epi-lipoxin A4 inhibits myeloperoxidase signaling and enhances resolution of acute lung injury. Am J Respir Crit Care Med. 2009;180(4):311–9.
Sunderland N, Hennessy A, Makris A. Animal models of pre-eclampsia. Am J Reprod Immunol. 2011;65(6):533–41.
Acknowledgments
The authors are grateful to Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES) Research Fellowship.
Author information
Authors and Affiliations
Corresponding author
Ethics declarations
Funding
This study was funded by Fundação de Amparo à Pesquisa do Estado de Minas Gerais (FAPEMIG; APQ-03318-15), Conselho Nacional de Desenvolvimento Científico e Tecnológico (CNPq; Research Fellowship), and Coordenação de Aperfeiçoamento de Pessoal de Nível Superior (CAPES; Ph.D. scholarship).
Conflict of interest
The authors declare that they have no conflict of interest.
Rights and permissions
About this article
Cite this article
Perucci, L.O., Corrêa, M.D., Dusse, L.M. et al. Resolution of inflammation pathways in preeclampsia—a narrative review. Immunol Res 65, 774–789 (2017). https://doi.org/10.1007/s12026-017-8921-3
Published:
Issue Date:
DOI: https://doi.org/10.1007/s12026-017-8921-3