-
PDF
- Split View
-
Views
-
Cite
Cite
Christophe Leclercq, Gabe B. Bleeker, Cecilia Linde, Erwan Donal, Jeroen J. Bax, Martin J. Schalij, Claude Daubert, Cardiac resynchronization therapy: clinical results and evolution of candidate selection, European Heart Journal Supplements, Volume 9, Issue suppl_I, December 2007, Pages I94–I106, https://doi.org/10.1093/eurheartj/sum066
Close - Share Icon Share
Abstract
Cardiac resynchronization therapy (CRT) is now a recommended treatment in patients with severe heart failure, a poor left ventricular (LV) ejection fraction and wide QRS of >120 ms. The implementation of CRT in the current guidelines for the treatment of chronic heart failure occurred after the results of different clinical trials including more than 4000 patients, especially the COMPANION and CARE-HF trials. These trials showed that CRT over and above optimal drug treatment improves symptoms, quality of life, exercise tolerance, but more importantly reduces mortality and morbidity. Another major finding was that CRT reverses LV remodelling, a major prognostic factor in heart failure. However, the rate of responders remains only 65–70%. So far, the only validated criterion of cardiac dyssynchrony is the QRS width on the surface ECG, which is probably not optimal. Echocardiographic techniques have shown interesting perspectives for optimizing patient selection, but still have to be validated. Moreover, new indications for CRT (patients with atrial fibrillation, narrow QRS, or mild heart failure) will probably be validated in the near future. The recent and future technical developments in the device will help to optimize the therapy and also to improve heart failure monitoring using sensors in the device and home monitoring by telephone or Internet.
Introduction
Cardiac resynchronization therapy (CRT) was introduced in the early 1990s and opened the era of the so-called electrical therapy for heart failure. At this time, pharmacological therapy had already dramatically improved the outcome of patients with systolic heart failure by a combination of rennin–angiotensin–aldosterone inhibitors and beta-blockers. Other pharmacological treatments in addition to these drugs have been evaluated, but results have been disappointing. In contrast, CRT in combination with pharmacological treatment significantly improved the outcome of severe heart failure patients. After rennin–angiotensin–aldosterone inhibitors and beta-blockers, CRT has been the most recent evolution and, even, revolution in the treatment of chronic heart failure. In this manuscript we will discuss the results of CRT, the current and future selection criteria for patients, who are candidates for CRT, and the future of CRT.
Clinical results of cardiac resynchronization therapy
In recent years CRT using simultaneous or sequential biventricular pacing has been proved an efficient adjunct therapy to optimal medical therapy in a subset of New York Heart Association (NYHA) III–IV heart failure patients. This benefit is based on the breakthrough work of randomized studies. The characteristics of patients in these studies1–7 are shown in Table 1. This clearly demonstrates that the evidence for patient benefit depends on severe heart failure symptoms, sinus rhythm, low left ventricular ejection fraction (LVEF) and prolongation of the QRS complex as a sign of ventricular dyssynchrony.
Symptomatic improvement
Importantly, the observation periods in the studies designed to measure symptomatic improvement such as the MUSTIC and MIRACLE were 3–6 months (Table 1 and 2).1–5 In most studies the 6 min walk distance was used to assess exercise capacity and the Minnesota Living with Heart Failure Questionnaire to assess quality of life. Functional capacity was assessed by the NYHA class, but in a few cases (MIRACLE and MIRACLE ICD studies) the global assessment as part of the clinical improvement score developed by Milton Packer was used.8 Although designed primarily as morbidity and mortality trials, symptom relief was also studied after 3–6 months in the CARE-HF and the COMPANION studies.6,7
The results of all these studies are in agreement and all demonstrate that CRT improves quality of life, exercise tolerance, and NYHA class compared with control treatment. Moreover, the CARE HF and COMPANION studies also indicate improvements in systolic blood pressure by CRT, which in turn may lead to an increased possibility of administration of heart failure medication in adequate dosage such as beta-blockers. Importantly, 30% of patients are non-responders as indicated in the MIRACLE-study,2 which is discussed elsewhere in this article.
Morbidity and mortality
The major cost in the management of heart failure remains hospitalization. From the MIRACLE study2 there were 25 hospitalizations in 228 patients in the CRT arm compared with 50 in 225 patients in the control arm. This study, however, was not powered to demonstrate differences in morbidity, which were instead studied in the COMPANION and CARE-HF.6,7
In the open COMPANION study 1520 patients were followed up for 15 months. All patients had been hospitalized at least once for heart failure within the last 12 months. Patients did not have a secondary indication for an implantable cardioverter defibrillator (ICD). Patients were randomized to CRT alone, CRT plus ICD (CRT-D), or medical therapy.6 The study was designed to compare the two device arms with medical therapy, but not to compare the results of CRT with CRT-D therapy. The primary endpoint of death or hospitalization from any cause was 56% in the CRT and CRT-D groups and 68% in the control group. This translates into a 34% relative reduction of the primary endpoint by CRT (P < 0.002) and a 40% reduction in the CRT-D arm (P < 0.001) compared with the medical therapy arm. All cause mortality after 12 months was 12% in the CRT-D, 15% in the CRT group, and 19% in the control group. This means a relative reduction of death of 24% (P = 0.059, NS) by CRT, and a 36% relative reduction by CRT-D (P = 0.003) compared with medical therapy.
The CARE-HF included 813 patients who were openly assigned to CRT or control therapy and followed up for 29.4 months.7 Most patients were on angiotensin-converting enzyme (ACE) inhibitors or angiotensin receptor blockers, and 75% were on beta-blockers. The total mortality after 12 months was 9.8% in the CRT arm and 12.6% in the control group, meaning in absolute terms 3% less risk of death in the CRT group. After extension to 37.4 month follow-up9 25% in the CRT compared with 38% in the control group had died indicating a progressively smaller risk of dying in the CRT group. Moreover, death because of heart failure was significantly reduced in the CRT group compared with control (HR 0.55, 95% CI 0.37–0.82; P = 0.003). Importantly and in contrast to the COMPANION study, with a much shorter follow-up sudden cardiac death was 7.8% in the CRT compared with 13.4 in the control arm (HR 0.54; P = 0.006).
This implies evidence that CRT reduces the risk for sudden cardiac death over time. The most probable explanation is reverse left ventricular (LV) remodelling, which can be observed as soon as 3 months after CRT and it develops fully over at least a 2 year period. It may be speculated that the addition of an ICD could further improve survival in patients treated with CRT. This remains to be established in a randomized study.
Reverse left ventricular remodelling
Although patients with pathological LV remodelling experience progressive worsening of heart failure, slowing or reversing remodelling has only recently been recognized as a goal of treatment.10 Reverse remodelling by ACE inhibitors and, in particular, beta-blockers11,12 have been linked to reduced morbidity and mortality in all classes of systolic heart failure. Reverse remodelling is a consistent finding in the CRT trials of patients with moderate-to-severe heart failure and wide QRS.13,14 Two relatively small studies indicate clinical improvements by CRT in NYHA class II patients,4,15 but no information to date exists concerning the possible benefits in patients with asymptomatic LV dysfunction and signs of ventricular dyssynchrony. The ongoing REVERSE and MADIT-CRT are expected to be the first to establish the value of CRT for slowing disease progression in such patients with asymptomatic LV dysfunction (but with previous symptoms) and in mild (NYHA II) heart failure.16,17
Meta-analysis of cardiac resynchronization therapy
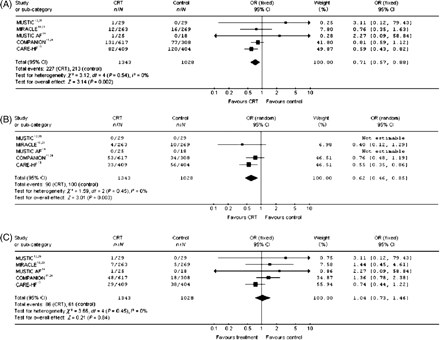
Four meta-analyses have demonstrated a statistically significant lower total mortality with CRT compared with controls.18–21 Mortality of heart failure was analysed in two of these studies and showed a significant reduction with CRT.18,22 In another study cardiovascular mortality was analysed and was not reduced by CRT.21 In the latest report sudden cardiac death mortality was also studied and found not to be significantly reduced by CRT18 (Figure 1). It is important to add that these meta-analyses are largely based on five randomized studies in which only the COMPANION and the CARE-HF have follow-up periods longer than 1 year. The patients in these two trials constitute 73% of the patient material in the meta-analyses in which the mean follow-up time was 18.4 months.
The predominant modes of death in heart failure are sudden cardiac death and heart failure. Patients with mild heart failure are more likely to die suddenly. A response to CRT will move the patients from NYHA III to NYHA II and, thus, increase the relative risk of dying suddenly. Thus, at present in patients with a life expectancy of at least 1 year, the available guidelines imply CRT-D to prevent the risk of sudden death.
To summarize, CRT improves symptoms and reduces morbidity and total mortality in patients with moderate-to-severe heart failure despite optimal heart failure medication with an ejection fraction below 35% and wide QRS as a sign of ventricular dyssynchrony. It remains to be established if this therapy is also efficient in preventing disease progression in mild heart failure and in patients with asymptomatic LV dysfunction.
Selection of candidates for cardiac resynchronization therapy: the present
European and US guidelines currently recommend CRT in a population of heart failure patients based on the inclusion criteria of the different clinical trials, which validated CRT.23,24 Four items characterized the potential population for CRT.
A criterion of severity of heart failure using the NYHA class.
A criterion of LV systolic dysfunction, the LVEF.
A criterion of the remodelling of the left ventricle, the LV end-diastolic diameter (LVEDD).
And finally a criterion of cardiac dyssynchrony based on the QRS duration on 12-lead surface ECG.
The severity of heart failure
The first implanted patients were patients with end-stage heart failure without other therapeutic approaches being available.25,26 Most of the clinical trials, which validated CRT included patients with moderate-to-severe heart failure. The MUSTIC trial included only NYHA class III patients, whereas the other trials except MIRACLE ICD and CONTAK-CD, which also included class II patients, were restricted to patients with NYHA class III or IV. However, in the various trials most of the patients were in NYHA class III: 91% in the MIRACLE population, 93% in CARE-HF, and 82% in COMPANION.1–7 The COMPANION trial probably included more severe patients because the inclusion criteria required a hospitalization for heart failure in the year previous to inclusion. So the results of these different trials are more accurate for NYHA class III patients and the benefit of CRT in NYHA class IV is still a matter of debate. A recent sub-analysis of the COMPANION trial showed that in ambulatory NYHA class IV patients, CRT significantly improved time to all cause mortality and hospitalizations with a trend towards improved mortality.27
The severity of left ventricular systolic dysfunction
All clinical trials included patients with LVEF below 35%. However, it is interesting to underline that the mean or median value of the LVEF in the trials was between 20 and 25%, suggesting that most of the included patients had very severe LV systolic dysfunction.1–7
The enlargement of the left ventricle
Cardiac resynchronization therapy focused on patients with a large left ventricle. To be included in these trials, an LVEDD of more than 55–60 or 30 mm indexed to height or body surface area. Generally, the left ventricle was much dilated with a mean LVEDD of 73 ± 10 mm in the MUSTIC trial and a median value of the LVEDD in the CARE-HF trial of 68 mm.1,7
The QRS duration
The current guidelines use as a marker of cardiac dyssynchrony, a wide QRS, more than 120 ms on the surface ECG.23,24 This cut-off value was chosen according to the inclusion criteria of the COMPANION and CARE-HF trials, which required a QRS width of at least 120 ms.6,7
The rationale for the selection of a wide QRS as a marker of cardiac dyssynchrony is based on the fact that electrical conduction disturbances enhance mechanical dyssynchrony28,29 with important modification of the timing of the periods in the cardiac cycle such as a prolongation of the isovolumic contraction and relaxation periods and LV ejection time and a dramatic decrease in LV filling time. A prolonged QRS duration, often associated with a prolongation of the atrio-ventricular interval, is present in 30–50% of heart failure patients with LV systolic dysfunction. These electrical disturbances, increasing over time and considered as independent predictors of major cardiac events and mortality, induce atrio-ventricular, interventricular, and intra-LV dyssynchronies.28 We must recognize that there are several issues with the QRS duration as a marker of cardiac dyssynchrony; the measurement of QRS width is theoretically easy but there are no specific recommendations. For instance, in the MUSTIC trial the mean value of the 12-leads was considered, whereas in the CARE-HF trial the presence QRS width greater than 120 ms was required in any two leads.1,7 Usually the accuracy of the measurement of QRS width is improved with a recording at 50 mm/s. Moreover, we must underline that if the cut-off value for QRS width is 120 ms, the mean or median value of the QRS duration of the patients included in the trials was 160 ms.1–7 For instance, in the CARE-HF trial where the patients might be included if they had either a QRS duration >150 ms or a QRS duration >120 ms and two criteria of echocardiographic mechanical dyssynchrony, only 8% with a QRS duration between 120 and 150 ms were included.7 So we can hypothesize that most of the patients included in these clinical trials had very wide QRS.
So far the responder rate to CRT remains stable and rather low around 55–70%.2,6,7 We can hypothesize that the selection of candidates for CRT using the ECG as a marker of cardiac dyssynchrony is probably not optimal. Different experimental or clinical studies showed that the correlation between electrical and mechanical is sometimes weak,30–32 and that some patients with wide QRS complexes may have no evidence of intra-LV asynchrony and that by contrast, some patients with ‘narrow’ QRS may exhibit mechanical dyssynchrony. The value of the QRS duration as a predictor of response to CRT is controversial. Most of the studies, and especially echocardiography-based studies, showed that baseline QRS duration is a weak predictor of response to CRT.33 However, all these studies included mostly patients with wide QRS with a mean value ranging from 170 to 190 ms. The reduction in QRS duration with biventricular pacing as a predictive factor of response to CRT is also controversial but was observed in many studies.33
Selection of candidates for cardiac resynchronization therapy: the next step?
The rate of non-responders to CRT in patients currently selected on a wide QRS as a marker of ventricular dyssynchrony raises the need for the refinement of the current selection criteria to (i) identify better those patients with the highest likelihood of response to CRT and (ii) avoid device implantations in patients that are unlikely to respond to CRT.
In addition, a better understanding of the beneficial mechanisms of CRT may lead to the identification of other patient groups that may potentially benefit from CRT but are not covered by current indications.
Why do patients benefit from CRT?
In heart failure patients with LV systolic dysfunction three types of mechanical dyssynchrony are frequently observed.28
Atrio-ventricular dyssynchrony, which results usually from the combination of a prolonged AV conduction and a widening of the QRS complex and mainly impairs diastolic filling.
Inter-ventricular dyssynchrony (dyssynchrony between the left and the right ventricles), which is usually the result of a delayed activation of the left ventricle.
LV dyssynchrony (dyssynchrony within the left ventricle), which is usually most prominent between the early activated inter-ventricular septum and the late-activated postero-lateral LV wall.
Through atrial synchronized biventricular stimulation, CRT is able to reduce all three types of cardiac dyssynchrony. However, recent studies now provide mounting evidence that the correction of LV dyssynchrony is the single most important beneficial mechanism of CRT.34–36 For example, Yu et al.35 demonstrated in 30 patients that those with severe LV dyssynchrony on echocardiography have a high likelihood of response following CRT, whereas patients without LV dyssynchrony did not improve after CRT. In contrast, baseline inter-ventricular dyssynchrony (between the left and the right ventricles) is usually similar between responders and non-responders.34–36
How to identify LV dyssynchrony?
Since the observation that QRS duration is a limited marker of LV dyssynchrony, several cardiac imaging techniques have been tested for their ability to quantify LV dyssynchrony and predict response to CRT. Among the different techniques, echocardiography proved particularly well suited to the detection of LV dyssynchrony in a clinical setting and the most important techniques will be discussed below.
M-mode echocardiography
One of the first and most simple techniques for the detection of LV dyssynchrony was introduced by Pitzalis et al.,37,38 who used M-mode echocardiography to measure LV dyssynchrony. In the parasternal short axis view (at the papillary muscle level) an M-mode recording is obtained through the interventricular septum and the posterior wall and the time delay in systolic excursion of both walls is measured as a marker of LV dyssynchrony (the so-called septal-to-posterior wall motion delay). In two subsequent studies including 80 patients, the authors found an optimal cut-off value of 130 ms to predict both an improvement in LVEF at 6 months follow-up and an improved long-term outcome (sensitivity 100% and a specificity 63%).37,38
The feasibility of this technique has recently been questioned by several studies that describe difficulties with the interpretation of the M-mode recordings in a high percentage of patients (up to 50%) because of a poor acoustic window in parasternal views or akinesia of the septum or posterior wall.39
Tissue Doppler imaging
At present, tissue Doppler imaging (TDI) is among the most widely studied techniques for the quantification of LV dyssynchrony. TDI is a derivation of the Doppler principle and is able to measure (the timing of) myocardial velocities among different LV segments. By calculating the differences between the time-to-peak systolic velocities among different LV segments, TDI proved an ideal tool to quantify LV dyssynchrony.
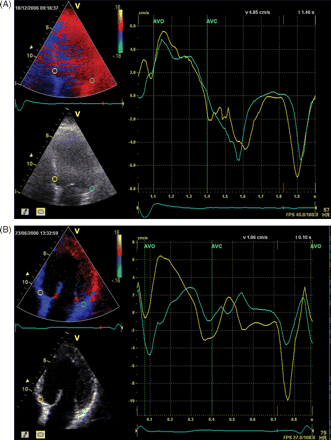
The myocardial velocity curves can be recorded either on-line from pulsed-wave TDI or reconstructed off-line from 2D colour-coded TDI images. The advantages of colour-coded TDI over pulsed wave TDI are greater accuracy of display of the peak systolic velocity and ability to measure multiple LV segments in one single heart beat. With colour-coded TDI most frequently two to four segments are measured (septum, anterior, lateral, and inferior) (Figure 2). Bax et al.34 evaluated 85 patients undergoing CRT implantation and demonstrated that LV dyssynchrony ≥65 ms among four basal segments was highly predictive for clinical improvement (sensitivity/specificity 80%) and LV reverse remodelling (sensitivity/specificity 92%). In addition, patients with baseline LV dyssynchrony ≥65 ms had a superior prognosis compared with patients without pre-implantation LV dyssynchrony.
Other studies have used a higher number of LV segments (6 or 12). For example, Yu et al.40 used colour-coded TDI to measure the time-to-peak systolic velocity among 12 LV segments (6 basal and 6 mid-LV segments) in 54 patients. The standard deviation of time-to-peak systolic velocity of the 12 segments was used as a marker of LV dyssynchrony, and a cut-off value of 31.4 ms was able to predict LV reverse remodelling at 3 months with a sensitivity of 96% and a specificity of 78%.40
An example of use of pulsed-wave TDI was published by Bordachar et al.,36 who included 41 patients undergoing CRT. In this study, LV dyssynchrony was defined as the greatest delay or standard deviation in peak or onset among 12 LV segments (6 basal and 6 mid-LV segments). Both the improvement in cardiac output and the reduction in mitral regurgitation following CRT were significantly correlated with the level of pre-implantation LV dyssynchrony.36
Recently, a novel development of TDI called tissue synchronization imaging has been introduced, which is able to display the time-to-peak systolic velocities as a colour map without the need for the calculation of the velocity curves. The early-activated segments are displayed in green and the latest activated segments displayed in orange/red. This technique allows for a quick visual assessment of LV dyssynchrony and initial studies have demonstrated promising results.41
Strain (rate) imaging
Strain (rate) imaging measures the (rate of) myocardial deformation and therefore has the potential advantage over TDI (which only measures myocardial velocity) to discriminate active from passive myocardial contraction. Most studies have applied strain (rate) imaging derived from TDI. For example, Breithardt et al.42 used strain (rate) imaging to evaluate the effects of CRT on regional LV strain (rate). In 18 heart failure patients the authors demonstrated that CRT is able acutely to reverse the septal–lateral difference in mid-segmental peak strain (from −46 ± 94 to 17 ± 92 ms P < 0.05). In a large proportion of patients the frequently observed phenomenon of early systolic wall lengthening of the septum and the late activation of the lateral wall was virtually eliminated by CRT.42 A recent study found an optimal cut-off value of 130 ms in difference in time-to-peak radial strain between the septum vs. the posterior wall (on the parasternal short axis), which was able to predict an immediate increase in stroke volume with a 95% sensitivity and 88% specificity.43
One of the problems with strain (rate) imaging derived from TDI is its relatively low reproducibility, which is mainly caused by the high angle dependency of this technique.40
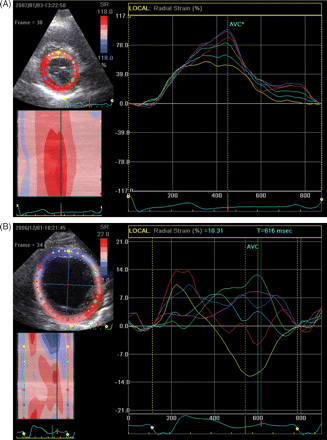
The problems with the reproducibility of strain (rate) measurements may potentially be overcome with the introduction of a new (angle-independent) technique, called speckle tracking, which is able to measure strain (rate) imaging from conventional 2D imaging. Suffoletto et al.44 recently tested this technique in 64 heart failure patients and demonstrated that a delay in time-to-peak strain in the radial direction of ≥130 ms predicted an acute increase in stroke volume (sensitivity 91% and specificity 75%) with CRT (Figure 3).
Three-dimensional echocardiography
Two recent studies have applied real-time three dimensional (3D) echocardiography for the assessment of LV dyssynchrony, which has the potential advantage over 2D techniques of being able to measure regional dyssynchrony of a large number of LV segments in one single heart beat.45
Using this technique the time to reach minimal regional volume can be calculated for each LV segment. LV dyssynchrony is defined as the standard deviation of the time to reach minimal regional LV volume for all 16 LV segments and is referred to as the systolic dyssynchrony index. Substantial LV dyssynchrony has been defined as a systolic dyssynchrony index of >3 SD above the mean for normal subjects (8.3%).45
In recent years many echocardiographic techniques have been introduced for the quantification of LV dyssynchrony in patients undergoing CRT ranging from simple M-mode echocardiography to more advanced echocardiographic techniques, such as TDI and 3D echocardiography. At present no consensus exists on the echocardiographic technique of choice and there is a clear need for larger multicentre trials directly comparing the different techniques to define a gold standard for echocardiographic LV dyssynchrony assessment. Interestingly, many small reports from single or two centres suggested that echocardiographic criteria and especially criteria using TDI would be powerful to predict positive response to CRT. These findings are of major interest, but these different echocardiographic techniques have not been validated in prospective trials in a large population of patients recruited from many centres. The PROSPECT trial designed to assess the potential role of different echocardiographic criteria to predict response to CRT may answer these questions.46 However, we must recognize that so far only the QRS duration as a marker of cardiac dyssynchrony has been evaluated and validated in clinical trials and is the only marker of dyssynchrony, currently in guidelines. New European guidelines will be published very soon and even if the value of echocardiography to assess cardiac dyssynchrony is discussed, echocardiographic criteria will not be introduced in these guidelines.
Emerging indications
CRT might be extended to other patients with respect to those considered eligible in the current guidelines.
Patients with permanent atrial fibrillation
Except the MUSTIC atrial fibrillation (AF) trial,3 all the clinical trials designed to evaluate CRT have included patients with stable sinus rhythm. However, in patients with moderate-to-severe heart failure, 20–30% have permanent AF. The results of the MUSTIC AF trial showed that in patients in whom CRT was delivered more than 85% of the time, biventricular pacing yielded significant improvements in symptoms, exercise tolerance, and quality of life compared with right ventricular pacing alone.3 A sub-analysis of the CARE-HF trial showed that in patients with new onset of AF, CRT significantly improved patient outcomes compared with optimal medical therapy.47 Interestingly, Gasparini et al.48 showed that in patients with permanent AF, the benefit observed with CRT was similar to that observed in sinus rhythm only in the sub-group of AF patients who underwent an atrio-ventricular node ablation and in whom biventricular pacing was applied at least 85% of time. Further large clinical trials specifically dedicated to this population are certainly warranted.
Patients with mild heart failure or asymptomatic left ventricular dysfunction
The MIRACLE ICD II and the CONTAK-CD trials also included patients with mild heart failure (NYHA class II).4,49 These trials showed that CRT did not improve symptoms or quality of life but had a positive effect on the LV reverse remodelling with a decrease in LV end-diastolic and end-systolic volumes and an increase in LVEF. The magnitude of benefits in LV reserve remodelling in less severe heart failure patients was similar to those observed in patients with moderate or severe heart failure (CARE-HF, MIRACLE). The REVERSE trial, specifically dedicated to patients with no or mild heart failure symptoms, has completed patient inclusion and the first results are expected in 2009 (16). Other trials such as the MADIT-CRT and the RAFT trials are currently ongoing and include also patients with NYHA class I or II.17
Patients with ‘narrow’ QRS
Several echocardiographic studies showed that 20–40% patients with ‘narrow’ QRS, i.e. a QRS duration less than 120 ms on surface ECG, have intra-LV dyssynchrony and would theoretically be candidates for CRT.30,31 Previous reports showed that CRT in NYHA class III or IV patients with an LVEF <35%, a QRS width less than 120 ms and evidence of inta-LV dyssynchrony significantly improved not only the cardiac synchrony but also symptoms, quality of life, and exercise tolerance.50,51 These encouraging results must be confirmed by prospective clinical trials. Several clinical trials are currently ongoing or starting to address this important question (RETHINQ, NARRO, ESTEEM, ECHOCRT, and others).
Patients with concomitant pacemakerindication
Right ventricular apical pacing, the most common pacing site, enhances cardiac dyssynchrony and has been shown to worsen patient outcomes.52 In patients with a high rate of ventricular pacing, different right ventricular pacing modalities are under evaluation such as alternatives RV pacing sites (septal, outflow tract, His or para-Hisian…), or biventricular pacing.52 The HOBIPACE trials in a small population suggested that CRT would be superior to conventional RV pacing.53 Other clinical trials aimed at addressing this issue further are ongoing such as the BIOPACE and BLOCK-HF trails.
Technological perspectives
What can we expect for the near future in CRT? The first step is probably to optimize the rate of responders. The rate of responders in the various clinical trials is stable and remains around 55–70% according to the criteria used to define responders.
There are interesting fields of research exploring the possibility to optimize the rate of response:
The selection of patients
The QRS duration is probably not the optimal criterion of cardiac dyssynchrony. Echocardiographic criteria are promising, but there is a lack of validation of these techniques. Other imaging techniques are also available such as magnetic resonance imaging, computed tomography scanning, radionuclide imaging techniques, or electro-mechanical mapping techniques, and might be helpful to optimize patient selection. The future is probably the fusion of data provided by these different techniques to identify cardiac dyssynchrony better combined with better selection of LV pacing sites.
The pacing site
At present, the LV pacing lead is positioned in the lateral or postero-lateral region of the left ventricle with the limitations of the coronary sinus anatomy and technical issues. Several studies have underlined the importance of the concordance between the pacing site and the site of the latest activation. To improve the LV lead implantation new technologies such as magnetic- or robotic-assisted navigation are currently being studied.
The optimization of the device
The programming of the device should be tailored for each patient, particularly the AV timing and possibly also the VV timing. However, optimization of these timings is often not performed mainly because of the time required. New features to determine automatically AV and VV timings, such as QUICK-OPT algorithm®, have recently been developed and are under evaluation. The next step would be a permanent and on-going optimization of the timing using a closed-loop system.
The LV pacing modalities
At present, the left ventricle is paced epicardially in one position. According to the complexity of LV dyssynchrony we can reasonably hypothesize that CRT would be improved if we would pace a larger area or more sites in the left ventricle. New technical solutions are currently under development to assess the feasibility and the efficacy of this pacing modality.
Cardiac resynchronization devices are not only designed to resynchronize the heart, but also to monitor different heart failure parameters. Indeed, CRT patients are primarily heart failure patients with an unstable haemodynamic condition. Different haemodynamic sensors (pressure, thoracic impedance, peak endocardial acceleration…) or heart failure markers (heart rate, activity sensors, heart rate variability, respiratory parameters…) may be implemented in devices to monitor the heart failure status of the patients. With the dramatic development of telemedicine and the potential of some parameters to detect heart failure deterioration prior to the occurrence of symptoms, we may reasonably expect to be warned of heart failure decompensation and so to reduce heart failure hospitalization. It is known that heart failure hospitalizations deteriorate quality of life, unfavourably impact the prognosis, and represent 70% of heart failure costs.
Most patients are implanted with a CRT-D device: more than 90% in USA and around 60–70% in Europe. The debate on the choice between CRT-P (pacing only) and CRT-D is largely open. However, one of the Achilles heels of defibrillation therapy is lead fracture, especially in young patients who are exposed to inappropriate shocks and inefficacy of the therapy. Some interesting research on lead-less ICDs is ongoing and could offer new interesting options in the future.
Clinical characteristics of patients in cardiac resynchronization therapy studies
| . | MUSTIC-SR, 20011 . | MIRACLE, 20022 . | MUSTIC-AF, (atr) 20023 . | CONTAK-CD, 20034 . | MIRACLE-ICD, 20035 . | COMPANION 20046 . | CARE-HF, 20057 . |
|---|---|---|---|---|---|---|---|
| Follow-up (months) | 3 | 6 | 3 | 3 | 6 | 12 | 12, 24, 29 |
| Age (mean ± SD) | 63 ± 10 | 64 ± 11 | 65 ± 8 | 66 ± 11 | 67 ± 10 | 67 | 67 |
| Women (%) | 26 | 32 | 19 | 16 | 23 | 33 | 26 |
| LVEF (mean ± SD) | 23 ± 7 | 22 ± 6 | 26 ± 10 | 22 ± 7 | 24 ± 6,5 | 21 | 25 (median) |
| QRS-width (mean ± SD) | 174 ± 20 | 166 ± 21 | 209 ± 18 | 158 ± 26 | 164 ± 22 | 160 | 160 (median) |
| Ischaemic heart disease (%) | 37 | 54 | ? | 69 | 70 | 55 | 38 |
| NYHA-class III–IV (%) | 100 | 100 | 100 | 68 | 100 | 87 | 100 |
| Quality of life MLHFQ (mean ± SD) | 47 ± 22 | 59 ± 21 | 44 ± 22 | 42 ± 23 | 56 ± 23 | Not performed | Not reported |
| Six minute walk (mean ± SD) | 350 ± 109 | 298 ± 93 | 329 ± 85 | 318 ± 120 | 243 ± 123 | 264 | Not reported |
| . | MUSTIC-SR, 20011 . | MIRACLE, 20022 . | MUSTIC-AF, (atr) 20023 . | CONTAK-CD, 20034 . | MIRACLE-ICD, 20035 . | COMPANION 20046 . | CARE-HF, 20057 . |
|---|---|---|---|---|---|---|---|
| Follow-up (months) | 3 | 6 | 3 | 3 | 6 | 12 | 12, 24, 29 |
| Age (mean ± SD) | 63 ± 10 | 64 ± 11 | 65 ± 8 | 66 ± 11 | 67 ± 10 | 67 | 67 |
| Women (%) | 26 | 32 | 19 | 16 | 23 | 33 | 26 |
| LVEF (mean ± SD) | 23 ± 7 | 22 ± 6 | 26 ± 10 | 22 ± 7 | 24 ± 6,5 | 21 | 25 (median) |
| QRS-width (mean ± SD) | 174 ± 20 | 166 ± 21 | 209 ± 18 | 158 ± 26 | 164 ± 22 | 160 | 160 (median) |
| Ischaemic heart disease (%) | 37 | 54 | ? | 69 | 70 | 55 | 38 |
| NYHA-class III–IV (%) | 100 | 100 | 100 | 68 | 100 | 87 | 100 |
| Quality of life MLHFQ (mean ± SD) | 47 ± 22 | 59 ± 21 | 44 ± 22 | 42 ± 23 | 56 ± 23 | Not performed | Not reported |
| Six minute walk (mean ± SD) | 350 ± 109 | 298 ± 93 | 329 ± 85 | 318 ± 120 | 243 ± 123 | 264 | Not reported |
Clinical characteristics of patients in cardiac resynchronization therapy studies
| . | MUSTIC-SR, 20011 . | MIRACLE, 20022 . | MUSTIC-AF, (atr) 20023 . | CONTAK-CD, 20034 . | MIRACLE-ICD, 20035 . | COMPANION 20046 . | CARE-HF, 20057 . |
|---|---|---|---|---|---|---|---|
| Follow-up (months) | 3 | 6 | 3 | 3 | 6 | 12 | 12, 24, 29 |
| Age (mean ± SD) | 63 ± 10 | 64 ± 11 | 65 ± 8 | 66 ± 11 | 67 ± 10 | 67 | 67 |
| Women (%) | 26 | 32 | 19 | 16 | 23 | 33 | 26 |
| LVEF (mean ± SD) | 23 ± 7 | 22 ± 6 | 26 ± 10 | 22 ± 7 | 24 ± 6,5 | 21 | 25 (median) |
| QRS-width (mean ± SD) | 174 ± 20 | 166 ± 21 | 209 ± 18 | 158 ± 26 | 164 ± 22 | 160 | 160 (median) |
| Ischaemic heart disease (%) | 37 | 54 | ? | 69 | 70 | 55 | 38 |
| NYHA-class III–IV (%) | 100 | 100 | 100 | 68 | 100 | 87 | 100 |
| Quality of life MLHFQ (mean ± SD) | 47 ± 22 | 59 ± 21 | 44 ± 22 | 42 ± 23 | 56 ± 23 | Not performed | Not reported |
| Six minute walk (mean ± SD) | 350 ± 109 | 298 ± 93 | 329 ± 85 | 318 ± 120 | 243 ± 123 | 264 | Not reported |
| . | MUSTIC-SR, 20011 . | MIRACLE, 20022 . | MUSTIC-AF, (atr) 20023 . | CONTAK-CD, 20034 . | MIRACLE-ICD, 20035 . | COMPANION 20046 . | CARE-HF, 20057 . |
|---|---|---|---|---|---|---|---|
| Follow-up (months) | 3 | 6 | 3 | 3 | 6 | 12 | 12, 24, 29 |
| Age (mean ± SD) | 63 ± 10 | 64 ± 11 | 65 ± 8 | 66 ± 11 | 67 ± 10 | 67 | 67 |
| Women (%) | 26 | 32 | 19 | 16 | 23 | 33 | 26 |
| LVEF (mean ± SD) | 23 ± 7 | 22 ± 6 | 26 ± 10 | 22 ± 7 | 24 ± 6,5 | 21 | 25 (median) |
| QRS-width (mean ± SD) | 174 ± 20 | 166 ± 21 | 209 ± 18 | 158 ± 26 | 164 ± 22 | 160 | 160 (median) |
| Ischaemic heart disease (%) | 37 | 54 | ? | 69 | 70 | 55 | 38 |
| NYHA-class III–IV (%) | 100 | 100 | 100 | 68 | 100 | 87 | 100 |
| Quality of life MLHFQ (mean ± SD) | 47 ± 22 | 59 ± 21 | 44 ± 22 | 42 ± 23 | 56 ± 23 | Not performed | Not reported |
| Six minute walk (mean ± SD) | 350 ± 109 | 298 ± 93 | 329 ± 85 | 318 ± 120 | 243 ± 123 | 264 | Not reported |
Results from randomized studies of CRT in patients with NYHA II-IV heart failure despite optimal medical therapy with LVEF ≤35% and QRS >120 ms
| Name . | Study design . | Study groups . | End points . | Results . |
|---|---|---|---|---|
| MUSTIC-SR Sinus rhythm, 20021 | Multicentre single blind crossover—3 months each study arm Comparison: CRT vs. non-CRT-VVI 40 | 58/67 included patients randomized and 48 completed crossover periods Lost: 9 in run in and 10 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | CRT improved 6 min walk by 23% (73 m) P < 0.001 Quality of life by MLHFQ improved 32% (−13.6 points) P < 0.001 |
| MIRACLE, 20022 | Multicentre double blind Parallel study Comparison: 6 months of CRT vs. 6 months of no CRT | 453/571 recruited patients were randomized CRT: 228 Control: 225 Lost: CRT: 13 Control: 24 | NYHA class, 6 min walk and quality of life were primary endpoints Hospitalizations for heart failure one of several secondary endpoints | NYHA class: CRT improved 68% whereas 30% were unchanged and 2% were worsened In control arm: 38% improved 58% were unchanged, and 4% worsened (P < 0.001) Six minute walk: Improved 39 m with CRT and 10 m without CRT (NS) Quality of life by MLHFQ: improved 18 points with CRT and 10 points without CRT (P = 0.02) Hospitalizations for heart failure were reduced by 34% with CRT and 18% without CRT (P = 0.02) |
| MUSTIC-AF Atrial fibrillation, 20023 | Multicentre crossover—3 months in each study arm Comparison: CRT on vs. off (VVI 70) | 43/64 recruited patients were randomized 39 completed crossover periods Lost: 21 in run in period 4 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | Six minute walk increased 18 m (P > 0.05) Quality of life by MLHFQ improved by 4.4 points (P > 0.05) |
| CONTAK-CD, 20034 | Multicentre crossover study Comparison: 3 months CRT on vs. off | 490/581 recruited patients were randomized and completed crossover periods Lost: 11 patients during run in period None during crossover periods | Maximal oxygen uptake, 6 min walk and quality of life were primary endpoints | Maximal oxygen uptake increased by 0.8 ml/kg/min (P = 0.03) Six minute walk distance increased 20 m (P = 0.043) |
| MIRACLE-ICD, 20035 | Multicentre double blind parallel study Comparison: 6 months of CRTon vs. off | 369/429 recruited patients were randomized 327 completed study protocol Intervention: 187 Control: 182 Lost: CRT: 10 Control: 14 got CRT | NYHA class, 6 min walk and quality of life were primary endpoints | NYHA class improved by 1 in CRT compared to 0 in control group (P = 0.007) Six minute walk: Increased by 55 m with CRT Increased by 53 m without CRT (P > 0.05) Quality of life MLHFQ increased by 17.5 points with CRT increase by 11 points without CRT (P = 0.02) |
| COMPANION, 20046 | Multicentre open parallel study Comparison: CRT/CRT-ICD or medical therapy alone in 2:2:1 fashion with 15 months follow-up | 1520 CRT 617 CRT-ICD595 Control 308 Lost: CRT 37 CRT-ICD 42 Control: 80 | Death and all cause mortality was the combined primary endpoint. Secondary endpoint: all cause mortality Six minute walk, quality of life and NYHA class assessed after 6 months | CRT and CRT-ICD reduced primary endpoint by 20 and 21%, respectively, compared with medical therapy (P = 0.01) Heart failures related death of hospitalizations were reduced compared with medical therapy by 34% in CRT arm (P < 0.001) and 40% in CRT-ICD arm (P < 0.001) Total mortality was reduced 24% in CRT arm (P = 0.059) and 36% in CRT-ICD arm (P < 0.003) Six minute walk increased by 40 m in both CRT arms (P < 0.001) NYHA class improved by 61% in CRT arms P < 0.001 |
| CARE-HF, 20057 | Multicentre open parallel study Comparison CRT vs. medical therapy with a 29.4 months follow-up | 814 patients were Randomized and completed protocol CRT: 409 Control: 404 Lost: CRT: 0 Control: 43 got CRT 23 got CRT-ICD | Primary endpoint: death or unplanned CV hospitalizations Quality of life and NYHA class evaluated after 3 months | Death or unplanned CV hospitalizations were 39% in CRT and 55% in control group P < 0.001 Total mortality was 20% in CRT and 30% in control group (P < 0.0001) Quality of life improved by 10 points P < 0.001 in CRT vs. control arm NYHA class improved 0.6 in CRT vs. control arm P < 0.001 |
| Name . | Study design . | Study groups . | End points . | Results . |
|---|---|---|---|---|
| MUSTIC-SR Sinus rhythm, 20021 | Multicentre single blind crossover—3 months each study arm Comparison: CRT vs. non-CRT-VVI 40 | 58/67 included patients randomized and 48 completed crossover periods Lost: 9 in run in and 10 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | CRT improved 6 min walk by 23% (73 m) P < 0.001 Quality of life by MLHFQ improved 32% (−13.6 points) P < 0.001 |
| MIRACLE, 20022 | Multicentre double blind Parallel study Comparison: 6 months of CRT vs. 6 months of no CRT | 453/571 recruited patients were randomized CRT: 228 Control: 225 Lost: CRT: 13 Control: 24 | NYHA class, 6 min walk and quality of life were primary endpoints Hospitalizations for heart failure one of several secondary endpoints | NYHA class: CRT improved 68% whereas 30% were unchanged and 2% were worsened In control arm: 38% improved 58% were unchanged, and 4% worsened (P < 0.001) Six minute walk: Improved 39 m with CRT and 10 m without CRT (NS) Quality of life by MLHFQ: improved 18 points with CRT and 10 points without CRT (P = 0.02) Hospitalizations for heart failure were reduced by 34% with CRT and 18% without CRT (P = 0.02) |
| MUSTIC-AF Atrial fibrillation, 20023 | Multicentre crossover—3 months in each study arm Comparison: CRT on vs. off (VVI 70) | 43/64 recruited patients were randomized 39 completed crossover periods Lost: 21 in run in period 4 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | Six minute walk increased 18 m (P > 0.05) Quality of life by MLHFQ improved by 4.4 points (P > 0.05) |
| CONTAK-CD, 20034 | Multicentre crossover study Comparison: 3 months CRT on vs. off | 490/581 recruited patients were randomized and completed crossover periods Lost: 11 patients during run in period None during crossover periods | Maximal oxygen uptake, 6 min walk and quality of life were primary endpoints | Maximal oxygen uptake increased by 0.8 ml/kg/min (P = 0.03) Six minute walk distance increased 20 m (P = 0.043) |
| MIRACLE-ICD, 20035 | Multicentre double blind parallel study Comparison: 6 months of CRTon vs. off | 369/429 recruited patients were randomized 327 completed study protocol Intervention: 187 Control: 182 Lost: CRT: 10 Control: 14 got CRT | NYHA class, 6 min walk and quality of life were primary endpoints | NYHA class improved by 1 in CRT compared to 0 in control group (P = 0.007) Six minute walk: Increased by 55 m with CRT Increased by 53 m without CRT (P > 0.05) Quality of life MLHFQ increased by 17.5 points with CRT increase by 11 points without CRT (P = 0.02) |
| COMPANION, 20046 | Multicentre open parallel study Comparison: CRT/CRT-ICD or medical therapy alone in 2:2:1 fashion with 15 months follow-up | 1520 CRT 617 CRT-ICD595 Control 308 Lost: CRT 37 CRT-ICD 42 Control: 80 | Death and all cause mortality was the combined primary endpoint. Secondary endpoint: all cause mortality Six minute walk, quality of life and NYHA class assessed after 6 months | CRT and CRT-ICD reduced primary endpoint by 20 and 21%, respectively, compared with medical therapy (P = 0.01) Heart failures related death of hospitalizations were reduced compared with medical therapy by 34% in CRT arm (P < 0.001) and 40% in CRT-ICD arm (P < 0.001) Total mortality was reduced 24% in CRT arm (P = 0.059) and 36% in CRT-ICD arm (P < 0.003) Six minute walk increased by 40 m in both CRT arms (P < 0.001) NYHA class improved by 61% in CRT arms P < 0.001 |
| CARE-HF, 20057 | Multicentre open parallel study Comparison CRT vs. medical therapy with a 29.4 months follow-up | 814 patients were Randomized and completed protocol CRT: 409 Control: 404 Lost: CRT: 0 Control: 43 got CRT 23 got CRT-ICD | Primary endpoint: death or unplanned CV hospitalizations Quality of life and NYHA class evaluated after 3 months | Death or unplanned CV hospitalizations were 39% in CRT and 55% in control group P < 0.001 Total mortality was 20% in CRT and 30% in control group (P < 0.0001) Quality of life improved by 10 points P < 0.001 in CRT vs. control arm NYHA class improved 0.6 in CRT vs. control arm P < 0.001 |
AF, atrial fibrillation; CRT, cardiac resynchronization therapy; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire (0–105 points—high value is poor quality of life); NS, not statistically significant change; SR, sinus rhythm; VVI 40, right ventricular pacing.
Results from randomized studies of CRT in patients with NYHA II-IV heart failure despite optimal medical therapy with LVEF ≤35% and QRS >120 ms
| Name . | Study design . | Study groups . | End points . | Results . |
|---|---|---|---|---|
| MUSTIC-SR Sinus rhythm, 20021 | Multicentre single blind crossover—3 months each study arm Comparison: CRT vs. non-CRT-VVI 40 | 58/67 included patients randomized and 48 completed crossover periods Lost: 9 in run in and 10 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | CRT improved 6 min walk by 23% (73 m) P < 0.001 Quality of life by MLHFQ improved 32% (−13.6 points) P < 0.001 |
| MIRACLE, 20022 | Multicentre double blind Parallel study Comparison: 6 months of CRT vs. 6 months of no CRT | 453/571 recruited patients were randomized CRT: 228 Control: 225 Lost: CRT: 13 Control: 24 | NYHA class, 6 min walk and quality of life were primary endpoints Hospitalizations for heart failure one of several secondary endpoints | NYHA class: CRT improved 68% whereas 30% were unchanged and 2% were worsened In control arm: 38% improved 58% were unchanged, and 4% worsened (P < 0.001) Six minute walk: Improved 39 m with CRT and 10 m without CRT (NS) Quality of life by MLHFQ: improved 18 points with CRT and 10 points without CRT (P = 0.02) Hospitalizations for heart failure were reduced by 34% with CRT and 18% without CRT (P = 0.02) |
| MUSTIC-AF Atrial fibrillation, 20023 | Multicentre crossover—3 months in each study arm Comparison: CRT on vs. off (VVI 70) | 43/64 recruited patients were randomized 39 completed crossover periods Lost: 21 in run in period 4 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | Six minute walk increased 18 m (P > 0.05) Quality of life by MLHFQ improved by 4.4 points (P > 0.05) |
| CONTAK-CD, 20034 | Multicentre crossover study Comparison: 3 months CRT on vs. off | 490/581 recruited patients were randomized and completed crossover periods Lost: 11 patients during run in period None during crossover periods | Maximal oxygen uptake, 6 min walk and quality of life were primary endpoints | Maximal oxygen uptake increased by 0.8 ml/kg/min (P = 0.03) Six minute walk distance increased 20 m (P = 0.043) |
| MIRACLE-ICD, 20035 | Multicentre double blind parallel study Comparison: 6 months of CRTon vs. off | 369/429 recruited patients were randomized 327 completed study protocol Intervention: 187 Control: 182 Lost: CRT: 10 Control: 14 got CRT | NYHA class, 6 min walk and quality of life were primary endpoints | NYHA class improved by 1 in CRT compared to 0 in control group (P = 0.007) Six minute walk: Increased by 55 m with CRT Increased by 53 m without CRT (P > 0.05) Quality of life MLHFQ increased by 17.5 points with CRT increase by 11 points without CRT (P = 0.02) |
| COMPANION, 20046 | Multicentre open parallel study Comparison: CRT/CRT-ICD or medical therapy alone in 2:2:1 fashion with 15 months follow-up | 1520 CRT 617 CRT-ICD595 Control 308 Lost: CRT 37 CRT-ICD 42 Control: 80 | Death and all cause mortality was the combined primary endpoint. Secondary endpoint: all cause mortality Six minute walk, quality of life and NYHA class assessed after 6 months | CRT and CRT-ICD reduced primary endpoint by 20 and 21%, respectively, compared with medical therapy (P = 0.01) Heart failures related death of hospitalizations were reduced compared with medical therapy by 34% in CRT arm (P < 0.001) and 40% in CRT-ICD arm (P < 0.001) Total mortality was reduced 24% in CRT arm (P = 0.059) and 36% in CRT-ICD arm (P < 0.003) Six minute walk increased by 40 m in both CRT arms (P < 0.001) NYHA class improved by 61% in CRT arms P < 0.001 |
| CARE-HF, 20057 | Multicentre open parallel study Comparison CRT vs. medical therapy with a 29.4 months follow-up | 814 patients were Randomized and completed protocol CRT: 409 Control: 404 Lost: CRT: 0 Control: 43 got CRT 23 got CRT-ICD | Primary endpoint: death or unplanned CV hospitalizations Quality of life and NYHA class evaluated after 3 months | Death or unplanned CV hospitalizations were 39% in CRT and 55% in control group P < 0.001 Total mortality was 20% in CRT and 30% in control group (P < 0.0001) Quality of life improved by 10 points P < 0.001 in CRT vs. control arm NYHA class improved 0.6 in CRT vs. control arm P < 0.001 |
| Name . | Study design . | Study groups . | End points . | Results . |
|---|---|---|---|---|
| MUSTIC-SR Sinus rhythm, 20021 | Multicentre single blind crossover—3 months each study arm Comparison: CRT vs. non-CRT-VVI 40 | 58/67 included patients randomized and 48 completed crossover periods Lost: 9 in run in and 10 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | CRT improved 6 min walk by 23% (73 m) P < 0.001 Quality of life by MLHFQ improved 32% (−13.6 points) P < 0.001 |
| MIRACLE, 20022 | Multicentre double blind Parallel study Comparison: 6 months of CRT vs. 6 months of no CRT | 453/571 recruited patients were randomized CRT: 228 Control: 225 Lost: CRT: 13 Control: 24 | NYHA class, 6 min walk and quality of life were primary endpoints Hospitalizations for heart failure one of several secondary endpoints | NYHA class: CRT improved 68% whereas 30% were unchanged and 2% were worsened In control arm: 38% improved 58% were unchanged, and 4% worsened (P < 0.001) Six minute walk: Improved 39 m with CRT and 10 m without CRT (NS) Quality of life by MLHFQ: improved 18 points with CRT and 10 points without CRT (P = 0.02) Hospitalizations for heart failure were reduced by 34% with CRT and 18% without CRT (P = 0.02) |
| MUSTIC-AF Atrial fibrillation, 20023 | Multicentre crossover—3 months in each study arm Comparison: CRT on vs. off (VVI 70) | 43/64 recruited patients were randomized 39 completed crossover periods Lost: 21 in run in period 4 during crossover periods | Six minute walk as primary and quality of life as secondary endpoint | Six minute walk increased 18 m (P > 0.05) Quality of life by MLHFQ improved by 4.4 points (P > 0.05) |
| CONTAK-CD, 20034 | Multicentre crossover study Comparison: 3 months CRT on vs. off | 490/581 recruited patients were randomized and completed crossover periods Lost: 11 patients during run in period None during crossover periods | Maximal oxygen uptake, 6 min walk and quality of life were primary endpoints | Maximal oxygen uptake increased by 0.8 ml/kg/min (P = 0.03) Six minute walk distance increased 20 m (P = 0.043) |
| MIRACLE-ICD, 20035 | Multicentre double blind parallel study Comparison: 6 months of CRTon vs. off | 369/429 recruited patients were randomized 327 completed study protocol Intervention: 187 Control: 182 Lost: CRT: 10 Control: 14 got CRT | NYHA class, 6 min walk and quality of life were primary endpoints | NYHA class improved by 1 in CRT compared to 0 in control group (P = 0.007) Six minute walk: Increased by 55 m with CRT Increased by 53 m without CRT (P > 0.05) Quality of life MLHFQ increased by 17.5 points with CRT increase by 11 points without CRT (P = 0.02) |
| COMPANION, 20046 | Multicentre open parallel study Comparison: CRT/CRT-ICD or medical therapy alone in 2:2:1 fashion with 15 months follow-up | 1520 CRT 617 CRT-ICD595 Control 308 Lost: CRT 37 CRT-ICD 42 Control: 80 | Death and all cause mortality was the combined primary endpoint. Secondary endpoint: all cause mortality Six minute walk, quality of life and NYHA class assessed after 6 months | CRT and CRT-ICD reduced primary endpoint by 20 and 21%, respectively, compared with medical therapy (P = 0.01) Heart failures related death of hospitalizations were reduced compared with medical therapy by 34% in CRT arm (P < 0.001) and 40% in CRT-ICD arm (P < 0.001) Total mortality was reduced 24% in CRT arm (P = 0.059) and 36% in CRT-ICD arm (P < 0.003) Six minute walk increased by 40 m in both CRT arms (P < 0.001) NYHA class improved by 61% in CRT arms P < 0.001 |
| CARE-HF, 20057 | Multicentre open parallel study Comparison CRT vs. medical therapy with a 29.4 months follow-up | 814 patients were Randomized and completed protocol CRT: 409 Control: 404 Lost: CRT: 0 Control: 43 got CRT 23 got CRT-ICD | Primary endpoint: death or unplanned CV hospitalizations Quality of life and NYHA class evaluated after 3 months | Death or unplanned CV hospitalizations were 39% in CRT and 55% in control group P < 0.001 Total mortality was 20% in CRT and 30% in control group (P < 0.0001) Quality of life improved by 10 points P < 0.001 in CRT vs. control arm NYHA class improved 0.6 in CRT vs. control arm P < 0.001 |
AF, atrial fibrillation; CRT, cardiac resynchronization therapy; LVEF, left ventricular ejection fraction; MLHFQ, Minnesota Living with Heart Failure Questionnaire (0–105 points—high value is poor quality of life); NS, not statistically significant change; SR, sinus rhythm; VVI 40, right ventricular pacing.
(A) Effect of cardiac resynchronization therapy alone vs. control on overall mortality. Reprinted with permission from European Heart Journal, Rivero-Ayerza et al.18 (B) Effect of cardiac resynchronization therapy alone vs. control on heart failure mortality. Reprinted with permission from European Heart Journal, Rivero-Ayerza et al.18 (C) Effect of cardiac resynchronization therapy alone vs. control on sudden cardiac death. Reprinted with permission from European Heart Journal, Rivero-Ayerza et al.18
Colour-coded tissue Doppler imaging in the apical four-chamber view. The myocardial velocity curves are derived from sample volumes that are placed off-line in the basal septum and the basal lateral wall (arrows indicate peak systolic velocities, E' and A' represent diastolic parameters). (A) Example of a patient without a time delay in peak systolic velocity between the septum and the lateral wall, indicating absence of left ventricular dyssynchrony. (B) Example of a patient with substantial left ventricular dyssynchrony between the peak systolic velocity of the septum (first arrow) and the lateral wall (second arrow).
Radial time-strain curves derived from speckle tracking. Radial strain is calculated from multiple circumferential points over the cardiac cycle. The curves are colour-coded in accordance with the segments on the parasternal short-axis view. (A) Example of a patient in whom the time to peak strain occurs simultaneously in all six segments, indicating the absence of left ventricular dyssynchrony (peak strain is indicated by the arrow). (B) Example of a patient with severe left ventricular dyssynchrony between the early-activated antero-septal (first arrow) and the late-activated postero-lateral segments (second arrow).
Conflict of interest: none declared.