-
PDF
- Split View
-
Views
-
Cite
Cite
Gordan Samoukovic, Tarek Malas, Benoit de Varennes, The role of pulmonary embolectomy in the treatment of acute pulmonary embolism: a literature review from 1968 to 2008, Interactive CardioVascular and Thoracic Surgery, Volume 11, Issue 3, September 2010, Pages 265–270, https://doi.org/10.1510/icvts.2009.228361
Close - Share Icon Share
Summary
Acute massive or submassive pulmonary embolism (PE) requires prompt diagnosis, risk-stratification and aggressive treatment. Mortality rates can rise up to 70% within the first hour of presentation and are strongly correlated with the degree of right ventricular (RV) dysfunction, cardiac arrest, and consequential congestive heart failure. While anticoagulation is universally employed, there are inadequate data to establish definitive guidelines for the management of massive PE despite the availability of multiple treatment modalities. Medical thrombolytic therapy has not been shown to significantly reduce mortality in patients with massive PE but is still widely employed, whereas surgical and catheter embolectomy are only reserved as last resort treatments for critically ill patients with hemodynamic instability, or for those who are either not candidates for or have failed thrombolysis. Following an extensive review of medical literature, we outline the treatment options for this clinical scenario while focusing on the role of surgical embolectomy. Although traditionally reserved as rescue therapy for cases of failed thrombolysis, surgical embolectomy is a safe procedure with low mortality when performed early and in a selected group of patients. Sufficient evidence exists to extend the criteria for surgical embolectomy from strictly rescue therapy to include hemodynamically stable patients with RV dysfunction. Multidisciplinary approach to this condition coupled with a meticulous surgical technique has significantly lowered the mortality associated with this surgical procedure over the last 10 years.
1. Introduction
Without prompt diagnosis and aggressive treatment, acute pulmonary embolism (PE) is frequently fatal. Massive PE, characterized by hemodynamic instability, and often occluding 50% or more of the pulmonary artery (PA) cross-sectional area, carries up to 70% mortality within the hour of presentation [1–3]. The International Cooperative Pulmonary Embolism Registry (ICOPER), which included 2452 patients from 52 centers in seven countries, suggests a 17.4% 90-day mortality among all patients suffering from a PE [4]. The same report suggests a 4.5% incidence of massive PE with a 52.4% associated mortality and a strong correlation with right ventricular (RV) dysfunction, RV thrombi, and congestive heart failure (CHF). Despite recent advances in both medical and surgical treatment strategies, no defined treatment algorithm exists for massive PE. While anticoagulation with heparin appears to be universally employed, the roles of thrombolysis, catheter, and surgical embolectomy are ill-defined [5]. To date, clot extraction and/or dissolution by either percutaneous or open-surgical technique has been reserved as a last resort treatment for hemodynamically unstable patients, often with severe RV dysfunction, or for those who are either not candidates for or have failed thrombolysis.
Acute massive or submassive PE remains a major therapeutic challenge with mortality of up to 60% in the first 6 h of the event [1, 2, 4, 5]. Once the patient has been hemodynamically stabilized, and in absence of contraindications to anticoagulation, almost universally, intravenous heparin is administered as a bolus dose followed by a continuous infusion to achieve appropriate clotting parameters. Some authorities recommend the addition of a thrombolytic agent even in cases where hemodynamic stability has been achieved. In cases of massive PE resulting in hemodynamic compromise (with or without RV dysfunction) refractory to conventional medical therapy, systemic and/or catheter-directed thrombolysis, catheter clot fragmentation and surgical embolectomy are the remaining therapeutic options.
2. Methods
In order to assess, the trends in current therapy for massive PE, an extensive literature search was performed using PubMed, which includes Medline, Ovid, and the Cochrane Library databases. The search was based on the following key terms: ‘embolectomy’, ‘pulmonary embolectomy’, ‘surgical embolectomy’, ‘catheter embolectomy’, ‘thrombolysis’, ‘catheter fragmentation’, and ‘embolus fragmentation’, all matched with ‘acute’ and ‘PE’. Case reports, studies with fewer than 10 patients, and animal studies as well as reports published prior to 1968 were excluded; the focus was on articles published since 1995. Among the 1742 reports identified, 291 were reviewed in detail leading to analysis of 50. The review of outcomes of surgical embolectomy by Stein et al. [6] was utilized to obtain surgical mortality rates quoted in several early reports; hence, some of the early studies were not cited separately.
This literature review revealed that indeed current treatment algorithms for massive PE at most centers advocate the use of thrombolytic therapy when not contraindicated, such as in cases of stroke, recent surgery or other forms of active bleeding. Most authorities agree that while thrombolysis and catheter thromboembolectomy have been effective in rapidly re-establishing hemodynamic stability, they are associated with a high incidence of hemorrhage (in particular, intracranial), fragment embolization to distal vasculature, leading to consequent pulmonary hypertension [1, 7, 8]. Traditionally, surgical embolectomy has been reserved for patients with massive PE who are either in cardiogenic shock or for those cases where less invasive measures (including thrombolysis) have failed or are contraindicated. Additionally, there appears to be a consensus that patients with free-floating thrombi in the right atrium or ventricle and those with paradoxical embolism through a patent foramen ovale should be treated surgically [5, 6]. The clear role of surgical embolectomy in the treatment algorithm for massive PE, however, is controversial and is most likely underestimated.
3. Discussion
There are only a limited number of studies describing outcomes of thrombolytic therapy for clinically significant PE. While ICOPER data for patients with massive PE revealed that thrombolysis did not reduce the 90-day mortality nor did it affect the rate of recurrence of PE, a meta-analysis of data from 11 randomized trials comparing thrombolytic therapy with only intravenous heparin in patients with acute PE might suggest otherwise [9]. Although this report by Wan et al. demonstrated that overall thrombolytic therapy did not significantly reduce mortality or PE recurrence [6.7% vs. 9.6%; odds ratio (OR) 0.67, 95% confidence interval (CI) 0.40–1.12, P for heterogeneity=0.48], a subgroup analysis of five of the trials that included patients with hemodynamic compromise demonstrated that compared with heparin, thrombolytic therapy significantly reduced both mortality and PE recurrence [9.4% vs. 19.0%; OR 0.45, 95% CI 0.22–0.92, number needed to treat (NNT)=10]. However, no benefit was demonstrated in the remaining six trials that excluded these patients. The same analysis determined that while seven of 11 trials suggested higher incidence of major bleeding in patients treated with thrombolysis, pooled data from all trials did not show such a trend (9.1% vs. 6.1%; OR 1.42, 95% CI 0.81–2.46). The incidence of minor bleeding, however, was uniformly increased among the patients treated with thrombolysis (22.7% vs. 10.0%; OR 2.63, 95% CI 1.53–4.54). The ICOPER data suggests a 21% and 3% incidence of major and intracranial or fatal hemorrhage, respectively. Additionally, pooled data from 312 patients in controlled studies regarding thrombolytic therapy reported by Proudfoot et al. revealed that these rates might be lower – 13% and 1.8%, respectively [5].
To date, very few studies have compared medical and surgical treatment options for PE in patients with hemodynamic instability. A study by Gulba et al. compared surgical embolectomy with thrombolysis among patients in cardiogenic shock secondary to massive PE; their analysis revealed that patients treated with thrombolytics had a higher mortality rate (33% vs. 23%), increased risk of major hemorrhage (25% vs. 15%), and higher PE recurrence rates (21% vs. 7.7%) [10, 11]. Although these reports rely on retrospective review of data and are subject to a significant element of selection bias and low study power in general, they at least challenge the benefits of thrombolytic therapy.
While thrombolysis has been shown to rapidly resolve clots in pulmonary vasculature, there are very few studies focusing on long-term outcomes of thrombolytic therapy. A study by Meneveau et al. of 249 patients with PE treated with thrombolysis demonstrated that, in the 227 patients who survived the acute phase of PE post-thrombolysis, the global survival rates decreased to 56% at a 10-year follow-up [12]. Further, the 36% of these patients who experienced PE-related events (recurrent deep vein thrombosis, recurrent PE, occurrence of CHF or change of New York Heart Association functional class to class III or IV) had significantly higher mortality than the rest. Several reports suggest that approximately 15% to 25% of patients have only partial resolution of pulmonary vasculature obstruction with distal embolization, resulting in persistent pulmonary hypertension and increased mortality [12]. Although there are no studies evaluating long-term outcomes of surgical embolectomy, Meneveau et al. report that in an acute setting, rescue surgical embolectomy post thrombolysis is superior to repeat thrombolysis. Specifically, the overall mortality (38% vs. 7%, P<0.05) and PE recurrence rates (35% vs. 0%, P<0.015) were significantly decreased [13]. While no significant difference in overall bleeding rates was observed in this study, all bleeding events in the repeat-thrombolysis group were fatal.
Catheter embolectomy is a novel therapeutic modality, and its role in the armamentarium for treatment of acute PE is also poorly defined. To date, it has most frequently been employed in cases where thrombolysis and/or surgical thromboembolectomy are contraindicated [14]. The American College of Chest Physicians suggests careful selection of patients for catheter embolectomy based on hemodynamic compromise, the degree of the filling defect in the PA, and contraindications to thrombolysis [14]. Although most contemporary catheters have been developed for coronary artery interventions, extensive work has been done on developing those suitable for pulmonary vasculature [15]. Nonetheless, catheter-based clot removal or dissolution is based on one of the three techniques: (1) clot fragmentation, (2) clot aspiration, and (3) rheolytic thrombectomy [16]. While the former two utilize the tip of the catheter itself to break apart or aspirate the clot, the latter depends on the Venturi effect created by a high-speed saline jet which fragments the clot. Clot fragmentation in any form increases the surface area of the clot itself, making it more susceptible to future thrombolysis while providing flow recovery and reduction in pulmonary vascular resistance leading to improvement of RV function.
No randomized control trials have been performed to assess the effectiveness of thrombus fragmentation with and without subsequent thrombolysis; hence, it is unclear whether adjuvant local or systemic thrombolysis should be administered. A single study compared the effects of localized catheter-delivered thrombolysis vs. systemic infusion of tPA and found no significant difference in outcomes [11]. Clinical experience with catheter embolectomy is extremely limited, leading to very few reported series and individual case studies. Early reports suggest the rate of successful clot extraction with meaningful recovery of RV function to be between 61% and 84% [17]. Skaff et al. reviewed the recent literature and reported success rates of catheter embolectomy with and without subsequent local or systemic thrombolysis to be 91% and 81% (Table 1 ), respectively, with minimal differences among various types of catheter employed [16]. A study by Kuo et al. of 12 patients with massive PE and hemodynamic instability revealed a success rate of 83% when catheter-directed intervention was employed; seven of these patients had failed prior thrombolysis and five had contraindications to thrombolytic therapy [19]. To date, no study comparing outcomes of surgical and catheter embolectomy exist; however, several case series and individual case reports describe use of surgical embolectomy as a rescue measure following unsuccessful catheter fragmentation with or without subsequent thrombolysis.
Success rates of catheter embolectomy with and without local and/or systemic thrombolysis. With any lysis, success rate was found to be 91% [18]
| Type of lysis | No | Systemic | Local | Local and |
| lysis | lysis | lysis | systemic lysis | |
| Number of patients | 108 | 30 | 171 | 39 |
| Success rate | 81% | 80% | 94% | 85% |
| Type of lysis | No | Systemic | Local | Local and |
| lysis | lysis | lysis | systemic lysis | |
| Number of patients | 108 | 30 | 171 | 39 |
| Success rate | 81% | 80% | 94% | 85% |
Success rates of catheter embolectomy with and without local and/or systemic thrombolysis. With any lysis, success rate was found to be 91% [18]
| Type of lysis | No | Systemic | Local | Local and |
| lysis | lysis | lysis | systemic lysis | |
| Number of patients | 108 | 30 | 171 | 39 |
| Success rate | 81% | 80% | 94% | 85% |
| Type of lysis | No | Systemic | Local | Local and |
| lysis | lysis | lysis | systemic lysis | |
| Number of patients | 108 | 30 | 171 | 39 |
| Success rate | 81% | 80% | 94% | 85% |
Although the incidence of insertion site bleeding is low (<10%), complications of catheter embolectomy are rarely reported but are potentially catastrophic, even in the hands of a skilled operator. These devices, initially developed for coronary or peripheral arteries, may cause injury to or perforation of central pulmonary vasculature leading to massive hemorrhage and inevitable death. Consequently, catheter embolectomy should be limited to main PAs and should not be used for clot extraction from lobar or smaller vessels.
Fragmentation theoretically leads to an increase in clot surface area and therefore more effective thrombolysis afterwards; however, it is unknown to what extent further dissolution of the clot occurs and on what time frame. Smaller clots lodged in segmental and subsegmental PAs may potentially lead to chronic obstructive embolic processes leading to chronic pulmonary hypertension and progressive RV dysfunction [8], which is correlated with decreased overall survival and adverse outcomes [1, 13]. Furthermore, recurrent embolism formation is more common after catheterization than after surgical embolectomy [1]. Other reported complications of this technique include RV and PA injury, arrhythmias from catheter passage through the right heart, acute pancreatitis, and severe hemolysis [1, 2, 5, 14]. Finally, most cases of catheter embolectomy are performed on an emergent basis with extremely low frequency in most centers; therefore, no single operator gains sufficient expertise at this intervention. Considering the complexity of potential complications and inability of interventional radiologists to manage them, no catheter embolectomy should be performed in absence of cardiothoracic surgical backup.
Recently, several major centers have liberalized the use of surgical embolectomy to include patients with PE associated with moderate-to-severe RV dysfunction without hemodynamic compromise [2, 20, 6]. RV dysfunction alone, documented by echocardiography, has been implicated as an early and late independent risk factor for RV failure and mortality in a number of studies [2, 12, 20–22], and recovery of its function has been identified as an early predictor of a favorable in-hospital course. Several recent retrospective reviews have addressed the role of surgical pulmonary embolectomy while emphasizing recent advances in diagnosis, surgical technique, and postoperative care. Leacche et al. suggest use of emergency embolectomy in patients with massive PE (defined by central clotting and hemodynamic instability), in those with hemodynamic stability with RV dysfunction as documented by echocardiography (submassive PE), and in patients with contra-indications to or failure of thrombolysis or percutaneous intervention [20]. This study retrospectively reviewed 47 patients who had undergone emergency surgical embolectomy and demonstrated significant reduction in both operative morbidity and mortality, favoring aggressive early surgical interventions. Aklog et al. and Digonnet et al. also considered hemodynamically stable patients with massive PE and moderate-to-severe RV dysfunction for surgical embolectomy [23, 24]. This former report of 29 patients reveals an impressive 30-day survival rate of 89%, but has included a smaller proportion of critically ill patients; in particular, only one patient suffering preoperative cardiac arrest underwent surgery.
Multiple surgical authorities have emphasized the critical importance of early surgical intervention; a study by Ahmed et al. revealed that patients who have undergone a surgical intervention in the first 24 h of the event experienced a 40% relative reduction in mortality rates [2]. Even though preoperative cardiac arrest requiring cardiopulmonary resuscitation has been identified as an independent risk factor causing mortality in up to 59% of patents with PE [3, 6], a recent report by Kadner et al. suggests a significant decrease in mortality even in this population of patients [25]. This is attributed to a multidisciplinary approach to diagnosis, treatment, and postoperative care.
While recent reports suggest the mortality from surgical embolectomy to be as low as 6%, statistical analysis of data published by Stein et al. and collected via our analysis of peer-reviewed literature suggests that mortality rates in patients treated with surgical embolectomy have indeed decreased substantially over time [6] (Table 2 ). Cumulative mortality within time periods 1968–1989, 1990–1999 and thereafter are calculated to be 35%, 30% and 19%, respectively (P<0.05).
Observed mortality rates in patients with massive pulmonary embolism
| Period | Lead author of the | Number of | Reported |
| identified report | cases | 30-day mortality | |
| 1968–1989 | Cooley [6] | 11 | 6/11 |
| Clarke [26] | 26 | 13/26 | |
| Heimbecker [6] | 11 | 1/11 | |
| Berger [18] | 17 | 4/17 | |
| Reul [27] | 17 | 6/17 | |
| De Weese [6] | 11 | 7/11 | |
| Miller [28] | 33 | 7/33 | |
| Tschirkov [6] | 24 | 7/24 | |
| Glassford [29] | 20 | 8/20 | |
| Botzauw [6] | 23 | 6/23 | |
| Mattox [30] | 39 | 22/39 | |
| Satter [31] | 46 | 17/46 | |
| Soyer [32] | 17 | 5/17 | |
| Savelyev [6] | 28 | 12/28 | |
| Clarke [26] | 55 | 20/55 | |
| Jaumin [33] | 23 | 7/23 | |
| Stalpaert [6] | 29 | 10/29 | |
| Lund [34] | 25 | 5/25 | |
| Gray [35] | 71 | 21/71 | |
| Total for period 1968–1989 | 526 | 184/526 (35.0%) | |
| 1990–1999 | Meyer [36] | 96 | 36/96 |
| Kieny [37] | 134 | 21/134 | |
| Boulafendis [6] | 16 | 5/16 | |
| Schmid [38] | 27 | 12/27 | |
| Bauer [39] | 44 | 9/44 | |
| Biglioli [6] | 11 | 3/11 | |
| Meyns [40] | 30 | 6/30 | |
| Leitz [41] | 13 | 6/13 | |
| Laas [42] | 34 | 15/34 | |
| Stulz [43] | 50 | 23/50 | |
| Gulba [10] | 13 | 3/13 | |
| Jakob [44] | 25 | 6/25 | |
| Doerge [45] | 36 | 9/36 | |
| Doerge [46] | 41 | 12/41 | |
| Chartier [47] | 17 | 8/17 | |
| Ullmann [48] | 40 | 14/40 | |
| Total for period 1990–1999 | 627 | 188/627 (30.0%) | |
| 2000–2008 | Dauphine [1] | 11 | 3/8 |
| Ahmed [2] | 15 | 12/15 | |
| Konstantinov [3] | 7 | 2/7 | |
| Yalamanchili [7] | 13 | 1/13 | |
| Leacche [20] | 47 | 3/47 | |
| Aklog [23] | 29 | 3/29 | |
| Digonnet [24] | 21 | 8/21 | |
| Kadner [25] | 25 | 2/25 | |
| Ando [49] | 15 | 4/15 | |
| Meneveau [13] | 14 | 1/14 | |
| Sukhija [22] | 18 | 2/18 | |
| Total for period 2000–2008 | 215 | 41/215 (19.1%) | |
| Period | Lead author of the | Number of | Reported |
| identified report | cases | 30-day mortality | |
| 1968–1989 | Cooley [6] | 11 | 6/11 |
| Clarke [26] | 26 | 13/26 | |
| Heimbecker [6] | 11 | 1/11 | |
| Berger [18] | 17 | 4/17 | |
| Reul [27] | 17 | 6/17 | |
| De Weese [6] | 11 | 7/11 | |
| Miller [28] | 33 | 7/33 | |
| Tschirkov [6] | 24 | 7/24 | |
| Glassford [29] | 20 | 8/20 | |
| Botzauw [6] | 23 | 6/23 | |
| Mattox [30] | 39 | 22/39 | |
| Satter [31] | 46 | 17/46 | |
| Soyer [32] | 17 | 5/17 | |
| Savelyev [6] | 28 | 12/28 | |
| Clarke [26] | 55 | 20/55 | |
| Jaumin [33] | 23 | 7/23 | |
| Stalpaert [6] | 29 | 10/29 | |
| Lund [34] | 25 | 5/25 | |
| Gray [35] | 71 | 21/71 | |
| Total for period 1968–1989 | 526 | 184/526 (35.0%) | |
| 1990–1999 | Meyer [36] | 96 | 36/96 |
| Kieny [37] | 134 | 21/134 | |
| Boulafendis [6] | 16 | 5/16 | |
| Schmid [38] | 27 | 12/27 | |
| Bauer [39] | 44 | 9/44 | |
| Biglioli [6] | 11 | 3/11 | |
| Meyns [40] | 30 | 6/30 | |
| Leitz [41] | 13 | 6/13 | |
| Laas [42] | 34 | 15/34 | |
| Stulz [43] | 50 | 23/50 | |
| Gulba [10] | 13 | 3/13 | |
| Jakob [44] | 25 | 6/25 | |
| Doerge [45] | 36 | 9/36 | |
| Doerge [46] | 41 | 12/41 | |
| Chartier [47] | 17 | 8/17 | |
| Ullmann [48] | 40 | 14/40 | |
| Total for period 1990–1999 | 627 | 188/627 (30.0%) | |
| 2000–2008 | Dauphine [1] | 11 | 3/8 |
| Ahmed [2] | 15 | 12/15 | |
| Konstantinov [3] | 7 | 2/7 | |
| Yalamanchili [7] | 13 | 1/13 | |
| Leacche [20] | 47 | 3/47 | |
| Aklog [23] | 29 | 3/29 | |
| Digonnet [24] | 21 | 8/21 | |
| Kadner [25] | 25 | 2/25 | |
| Ando [49] | 15 | 4/15 | |
| Meneveau [13] | 14 | 1/14 | |
| Sukhija [22] | 18 | 2/18 | |
| Total for period 2000–2008 | 215 | 41/215 (19.1%) | |
Observed mortality rates in patients with massive pulmonary embolism
| Period | Lead author of the | Number of | Reported |
| identified report | cases | 30-day mortality | |
| 1968–1989 | Cooley [6] | 11 | 6/11 |
| Clarke [26] | 26 | 13/26 | |
| Heimbecker [6] | 11 | 1/11 | |
| Berger [18] | 17 | 4/17 | |
| Reul [27] | 17 | 6/17 | |
| De Weese [6] | 11 | 7/11 | |
| Miller [28] | 33 | 7/33 | |
| Tschirkov [6] | 24 | 7/24 | |
| Glassford [29] | 20 | 8/20 | |
| Botzauw [6] | 23 | 6/23 | |
| Mattox [30] | 39 | 22/39 | |
| Satter [31] | 46 | 17/46 | |
| Soyer [32] | 17 | 5/17 | |
| Savelyev [6] | 28 | 12/28 | |
| Clarke [26] | 55 | 20/55 | |
| Jaumin [33] | 23 | 7/23 | |
| Stalpaert [6] | 29 | 10/29 | |
| Lund [34] | 25 | 5/25 | |
| Gray [35] | 71 | 21/71 | |
| Total for period 1968–1989 | 526 | 184/526 (35.0%) | |
| 1990–1999 | Meyer [36] | 96 | 36/96 |
| Kieny [37] | 134 | 21/134 | |
| Boulafendis [6] | 16 | 5/16 | |
| Schmid [38] | 27 | 12/27 | |
| Bauer [39] | 44 | 9/44 | |
| Biglioli [6] | 11 | 3/11 | |
| Meyns [40] | 30 | 6/30 | |
| Leitz [41] | 13 | 6/13 | |
| Laas [42] | 34 | 15/34 | |
| Stulz [43] | 50 | 23/50 | |
| Gulba [10] | 13 | 3/13 | |
| Jakob [44] | 25 | 6/25 | |
| Doerge [45] | 36 | 9/36 | |
| Doerge [46] | 41 | 12/41 | |
| Chartier [47] | 17 | 8/17 | |
| Ullmann [48] | 40 | 14/40 | |
| Total for period 1990–1999 | 627 | 188/627 (30.0%) | |
| 2000–2008 | Dauphine [1] | 11 | 3/8 |
| Ahmed [2] | 15 | 12/15 | |
| Konstantinov [3] | 7 | 2/7 | |
| Yalamanchili [7] | 13 | 1/13 | |
| Leacche [20] | 47 | 3/47 | |
| Aklog [23] | 29 | 3/29 | |
| Digonnet [24] | 21 | 8/21 | |
| Kadner [25] | 25 | 2/25 | |
| Ando [49] | 15 | 4/15 | |
| Meneveau [13] | 14 | 1/14 | |
| Sukhija [22] | 18 | 2/18 | |
| Total for period 2000–2008 | 215 | 41/215 (19.1%) | |
| Period | Lead author of the | Number of | Reported |
| identified report | cases | 30-day mortality | |
| 1968–1989 | Cooley [6] | 11 | 6/11 |
| Clarke [26] | 26 | 13/26 | |
| Heimbecker [6] | 11 | 1/11 | |
| Berger [18] | 17 | 4/17 | |
| Reul [27] | 17 | 6/17 | |
| De Weese [6] | 11 | 7/11 | |
| Miller [28] | 33 | 7/33 | |
| Tschirkov [6] | 24 | 7/24 | |
| Glassford [29] | 20 | 8/20 | |
| Botzauw [6] | 23 | 6/23 | |
| Mattox [30] | 39 | 22/39 | |
| Satter [31] | 46 | 17/46 | |
| Soyer [32] | 17 | 5/17 | |
| Savelyev [6] | 28 | 12/28 | |
| Clarke [26] | 55 | 20/55 | |
| Jaumin [33] | 23 | 7/23 | |
| Stalpaert [6] | 29 | 10/29 | |
| Lund [34] | 25 | 5/25 | |
| Gray [35] | 71 | 21/71 | |
| Total for period 1968–1989 | 526 | 184/526 (35.0%) | |
| 1990–1999 | Meyer [36] | 96 | 36/96 |
| Kieny [37] | 134 | 21/134 | |
| Boulafendis [6] | 16 | 5/16 | |
| Schmid [38] | 27 | 12/27 | |
| Bauer [39] | 44 | 9/44 | |
| Biglioli [6] | 11 | 3/11 | |
| Meyns [40] | 30 | 6/30 | |
| Leitz [41] | 13 | 6/13 | |
| Laas [42] | 34 | 15/34 | |
| Stulz [43] | 50 | 23/50 | |
| Gulba [10] | 13 | 3/13 | |
| Jakob [44] | 25 | 6/25 | |
| Doerge [45] | 36 | 9/36 | |
| Doerge [46] | 41 | 12/41 | |
| Chartier [47] | 17 | 8/17 | |
| Ullmann [48] | 40 | 14/40 | |
| Total for period 1990–1999 | 627 | 188/627 (30.0%) | |
| 2000–2008 | Dauphine [1] | 11 | 3/8 |
| Ahmed [2] | 15 | 12/15 | |
| Konstantinov [3] | 7 | 2/7 | |
| Yalamanchili [7] | 13 | 1/13 | |
| Leacche [20] | 47 | 3/47 | |
| Aklog [23] | 29 | 3/29 | |
| Digonnet [24] | 21 | 8/21 | |
| Kadner [25] | 25 | 2/25 | |
| Ando [49] | 15 | 4/15 | |
| Meneveau [13] | 14 | 1/14 | |
| Sukhija [22] | 18 | 2/18 | |
| Total for period 2000–2008 | 215 | 41/215 (19.1%) | |
Given the clinical and logistical difficulties in this patient population, recommendations for treatment of PE will likely never be based on a randomized clinical trial, particularly for critically ill patients. Thrombolytic therapy has been in use for acute coronary events for several decades, yet a definitive evaluation of its role (if any) in the treatment of pulmonary thrombotic events is long overdue [50]. Multiple studies confirm that surgical embolectomy is a successful treatment strategy with low mortality and morbidity rates, which have substantially improved with new treatment strategies, including a multidisciplinary approach to rapid diagnosis by computed tomography and echocardiography and prompt surgical intervention. When compared to medical treatment of massive PE, particularly in the last decade, surgical embolectomy was found to have lower mortality rates, a lower number of hemorrhagic events and recurrent thrombosis [25]. We present a case of successful surgical treatment of a patient with a massive pulmonary embolus complicated with hemodynamic collapse and RV dysfunction. This success was based on rapid diagnosis, prompt decision-making to proceed with the surgical intervention and efficient postoperative care. Safe perioperative course was ensured by a surgical approach involving cardiopulmonary bypass with mild hypothermia providing careful unloading and resuscitation of the RV, complete clot extraction via bilateral lung compressions, absence of aortic clamping and placement of an inferior vena cava (IVC) filter. Although the role of the IVC filters has been a controversial issue, ICOPER data as well as several recent studies favor its use. Postoperative management with milrinone and/or nitric oxide aimed at improving RV function was of paramount importance.
4. Conclusion
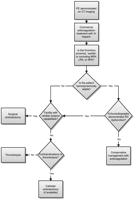
Recent advances in modern surgical techniques, coupled with a multidisciplinary approach to diagnosis and perioperative care, have significantly decreased the morbidity and mortality associated with surgical pulmonary embolectomy. Although its role in the armamentarium of treatment options for PE has not been clearly defined, sufficient evidence exists to expand the inclusion criteria for this surgical procedure from strictly rescue therapy for hemodynamically unstable patients to those who show extensive clotting affecting the RV function in the absence of hemodynamic collapse. We propose an algorithm for the management of a patient with massive or submassive PE in Fig. 1 . Considering the extremely high early mortality in cases of massive and submassive PE, decision for surgical intervention should be made quickly without wasting valuable time considering treatment options with lower rates of success and potentially detrimental complications.
Proposed algorithm for treatment of acute massive/submassive PE.
Presented at the 59th International Congress of the European Society for CardioVascular Surgery, Izmir, Turkey, April 15–18, 2010.
References
- anticoagulation
- cardiac arrest
- thrombolytic therapy
- embolectomy
- pulmonary embolism, massive, acute
- congestive heart failure
- critical illness
- heart ventricle
- surgical procedures, operative
- diagnosis
- guidelines
- mortality
- catheters
- hemodynamic instability
- pulmonary embolectomy
- stratification
- medical literature
- subacute massive pulmonary embolism