Abstract
The prescription of pharmaceuticals in the critically ill is complicated by a paucity of knowledge concerning the pharmacokinetic implications of the underlying disease state. Changes in organ function can be dramatic in this population, both as a consequence of the primary pathophysiology and in response to clinical interventions provided. Vascular tone, fluid status, cardiac output and major organ blood flow can be significantly altered from baseline, influencing the volume of distribution and clearance of many commonly prescribed agents.
Although measurable endpoints can be used to titrate doses for many drugs in this setting (such as sedatives), for those agents with silent pharmacodynamic indices, enhanced excretory organ function can result in unexpectedly low plasma concentrations, leading to treatment failure. This is particularly relevant to the use of antibacterials in the critically ill, where inadequate, inappropriate and/or delayed prescription can have significant effects on morbidity and mortality.
Augmented renal clearance (ARC) refers to enhanced renal elimination of circulating solute and is being described with increasing regularity in the critically ill. However, defining this process in terms of current measures of renal function is problematic, as although the glomerular filtration rate (GFR) is largely considered the best index of renal function, there is no consensus on an upper limit of normal. In addition, the most readily available and accurate estimate of the GFR at the bedside is still widely debated. From a pharmacokinetic point of view, ARC can result in elevated renal elimination and subtherapeutic plasma concentrations of pharmaceuticals, although whether this process solely involves augmented filtration (as opposed to enhanced tubular secretion and/or reabsorption) remains uncertain.
The primary contributors to this process are likely to be the innate immune response to infection and inflammation (with its associated systemic and haemodynamic consequences), fluid loading and use of vasoactive medications. The resultant increase in cardiac output and renal blood flow prompts enhanced glomerular filtration and drug elimination. Current evidence suggests that young patients without preexisting co-morbidity or organ dysfunction who present with trauma are most likely to manifest ARC. As this phenomenon has received little attention in the literature, dose modification has rarely been considered.
However, with increasing data supporting the concept, and many investigators demonstrating subtherapeutic concentrations of drugs in the critically ill, consideration of ARC and alternative dosing regimens is now mandatory, both to improve the likelihood of treatment success and to reduce the rate of development of antibacterial resistance.
Similar content being viewed by others
Augmented renal clearance (ARC) in patients without organ dysfunction is being increasingly described in subsets of critically ill patients.[1–11] In the context of antibacterial therapy, ARC has the potential to result in subtherapeutic dosing, treatment failure or selection of resistant micro-organisms.[11,12] This has significant implications in patients with sepsis, whereby the consequences of inappropriate antibacterial therapy may be catastrophic.[13–16] Given the persisting high associated intensive care unit (ICU) and in-hospital mortality rates,[17] action against any pathophysiology that alters antibacterial efficacy should be considered mandatory. ARC is also likely to be a key mechanism underlying the high antibacterial clearances previously described in patients with significant burn injury[4,18–24] or haematological malignancy.[25–28]
Current evidence stresses that the prescription of antibacterials in critically ill patients with sepsis is complex. Pharmacokinetic variability may be significant, with fluid shifts, altered capillary permeability, impaired vascular tone, organ dysfunction and multi-organ failure all likely to alter the pharmacokinetics of many routinely prescribed agents.[29] Although ARC is a factor rarely considered in this context, it increases the likelihood of suboptimal antibacterial concentrations.
The aims of this article are to define ARC, review its significance in the critically ill and explore the underlying mechanisms within the context of methods commonly used to assess renal function. We also seek to describe those subpopulations of critically ill patients most ‘at risk’ from ARC and review the implications for antibacterial dosing strategies in these groups.
Data for this review were identified by searches of MEDLINE (from 1966 to January 2009) and EMBASE (from 1966 to January 2009). The search terms included ‘creatinine clearance’, ‘antibiotics’, ‘pharmacokinetics’ and ‘critical illness’. In addition, other references were identified from the extensive files of the authors and from reference lists of identified papers.
1. What is Augmented Renal Clearance (ARC)?
A key function of the human kidney is excretion of circulating metabolites, toxins, waste products and pharmaceuticals, through a combination of glomerular filtration, tubular secretion and reabsorption. ARC refers to enhanced elimination of solute as compared with an expected baseline. However, accurately defining this process is problematic and is reliant on the accepted ‘normal’ values of renal function for a particular patient or population. The most widely accepted descriptor of renal function in health and disease is the glomerular filtration rate (GFR), and ‘normal’ values are roughly 130 mL/min/1.73 m2 and 120 mL/min/1.73 m2 in young men and young women, respectively.[30] Importantly, these values decline with age[30] (figure 1). Previously, some authors have attempted to define abnormal glomerular filtration on the basis of elevated GFRs.
Normal values of the glomerular filtration rate (GFR) in men and women. Inulin clearance for various ages in mL/min/1.73 m2 (a) in men and (b) in women. The solid lines represent the mean values of the GFR per decade of age, and the dashed lines represent the values of 1 standard deviation from the mean. (Reproduced from Stevens et al.,[30] with permission.)
Sunder-Plassmann and Horl[31] have proposed a categorization system for elevated GFRs, which they term ‘glomerular hyperfiltration’. In their article, the authors defined glomerular hyperfiltration on the basis of a GFR ≥120 mL/min/1.73 m2 (>149 mL/min/1.73 m2 in young adults). However, this categorization system does not differentiate between the sexes, requires further validation in the critically ill and may represent values within the normal range for some patients. In addition, the term ‘glomerular hyperfiltration’ describes changes typically seen in chronic kidney disease, and may not be representative of the mechanisms in critical illness.
Our proposed definition of ARC uses values 10% above the upper limit of normal — namely, GFRs >160 mL/min/1.73 m2 in men and >150 mL/min/1.73 m2 in women. Albeit conservative, these cutoffs are more likely to identify patients with truly augmented clearances. In addition, as a number of separate authors have identified creatinine clearance (CLCR) as a key pharmacokinetic covariate in predicting the clearance of many antibacterials,[5,32–41] this definition is an important step in identifying subpopulations at risk of subtherapeutic antibacterial exposure. Further work is clearly needed to refine these values and correlate them with pharmacokinetic and outcome data.
1.1 Physiological Changes in Critical Illness Likely to Contribute to ARC
The systemic inflammatory response syndrome (SIRS), a part of the innate immune response, describes a syndrome of physiological and laboratory derangements that can be recognized in the critically ill, regardless of the underlying aetiology.[42] Potential causes include trauma,[43] pancreatitis, burn injury, autoimmune disorders, ischaemia and major surgical procedures.[44] Sepsis is then defined as the presence of infection in conjunction with SIRS.[45]
The primary haemodynamic manifestations include a low systemic vascular resistance index and high cardiac output.[46] The underlying hypermetabolic and inflammatory state is driven by the release of endogenous cytokines and inflammatory mediators, in addition to the relative cellular dysoxia. How these changes impact on renal function is still being studied. In large animal models of sepsis, renal blood flow has been documented to increase in concert with cardiac output,[47] and in postoperative critically ill patients, cardiac output has been closely correlated with CLCR.[3]
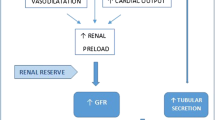
Measures to improve cardiovascular function in the critically ill commonly involve administration of intravenous resuscitation fluids and use of vasoactive medications.[48,49] Animal research has confirmed that crystalloid administration can result in an increase in CLCR,[50] although the influence of vasoactive agents on renal blood flow is continuing to be investigated.[51] Recent experimental data suggest that norepinephrine (noradrenaline) [a commonly employed vasopressor/inotrope] acts to increase cardiac output,[52,53] renal blood flow[52] and CLCR,[52,53] particularly in states characterized by marked vasodilation.[54] Previous studies in human sepsis and septic shock have confirmed a positive effect of norepinephrine on CLCR.[55–57] Figure 2 schematically illustrates the potential mechanisms underlying ARC in the critically ill.
These data suggest that in critically ill patients without significant renal dysfunction and in whom adequate resuscitation has been achieved, ARC is likely to be common. However, many patients can develop acute kidney injury with sepsis, secondary to mechanisms that are still being investigated,[58] and often in concert with significant laboratory and physiological derangement.[59] As a consequence, consideration of dose reduction of drugs in this setting may also be necessary.
1.2 Assessing the Presence of ARC in the Critically Ill
The most accurate, routinely available method of assessing the GFR is still uncertain, although serum creatinine concentrations are routinely used as an index of glomerular filtration in a clinical setting. However, isolated serum creatinine concentrations within the ‘normal’ reference range are insensitive indicators of the GFR in the ICU.[60] In addition, age, sex, race, state of hydration, muscle mass, metabolic state and muscle injury may all influence this value.[61] Despite these limitations, and perhaps incorrectly, acute elevations in serum creatinine concentrations are routinely interpreted as renal dysfunction, particularly in concert with oliguria (urine output <0.5 mL/kg/h).
In contrast, a ‘normal’ serum creatinine concentration within the laboratory reference range is assumed to equate to normal renal function, especially when the urine output is greater than 0.5 mL/kg/h. However, this interpretation of renal function may not always be correct. In patients whose protein stores or intake may be low, such as those who are elderly, malnourished, debilitated, hospitalized for long periods, or at the end of pregnancy, a ‘normal’ serum creatinine concentration may represent significant renal impairment. Recent work has also suggested that low baseline serum creatinine concentrations may be a risk factor for mortality in the critically ill.[62]
Numerous equations have been developed to estimate the GFR from serum creatinine values in ambulatory or ward-based patients with chronic kidney disease. The Modification of Diet in Renal Disease (MDRD) equation[63] was developed using data from 1628 patients with chronic kidney disease and calculates an estimated GFR adjusted to body surface area. Although it is a useful tool to screen for and monitor chronic kidney disease, its main limitation is in those without renal impairment, where inaccuracies have been reported at higher GFRs.[64–66] The Cockcroft-Gault equation[67] was developed in 1973 in 249 male patients and, although widely employed, has poor application in the critically ill.[60,68–70] Using historical data, Jelliffe[71] has proposed a method of estimating CLCR in those with unstable renal function. Although of significant merit, this method includes a requirement for at least two serum creatinine values (usually separated by 24 hours), and it suffers from the pitfalls of measuring serum creatinine concentrations in the critically ill. Further validation in this setting is needed.
Although these equations potentially provide more useful data than serum creatinine concentrations alone, particularly in patients recently admitted to the ICU,[72] clinicians should be dissuaded from using such equations to calculate the GFR, as they ignore the important effects of disease pathophysiology. Urinary creatinine collections of 8, 12 and 24 hours have been used to determine the GFR in critically ill patients,[73–75] although a 2-hour collection may be just as accurate.[9,72] Some authors have advocated using longer collection periods to improve the accuracy of GFR estimates[76] although, given the dynamic nature of critical illness, controversy exists over the most useful time for specimen collection. This is compounded by the circadian nature of the GFR,[77] and intraindividual variability is likely to be substantial. More frequent measurement is suggested in the critically ill, as there is potential for rapid changes in organ function.
Other markers such as inulin,[78] sinistrin[79] and cystatin C[30,80] may also have benefits for estimating the GFR but have not been widely adopted in clinical practice. Thus, because of the established correlation with drug clearance[5,7] and ease of measurement in the ICU, we believe that a timed urinary creatinine collection remains the most appropriate and convenient method for identifying patients with ARC.
1.3 ARC versus Renal Tubular Secretion or Reabsorption
Although it is convenient to define ARC on the basis of an elevated GFR, it is unknown whether there is a concomitant change in tubular secretion or reabsorption. Assessment of either component of renal function remains a difficult task, particularly as there is no specific agreed test that evaluates each process simultaneously or is routinely available. In a research context, this has prompted the administration of a ‘cocktail’ of different markers to characterize each process in the individual patient.[81,82] Future work is urgently needed in this area to outline any role in the critically ill.
1.4 Prevalence of ARC and Subpopulations Likely to Develop It
Although few robust studies are available, some data exist that may be instructive as to the prevalence of ARC as well as the patients most at risk. In a single-centre ICU observational study, Fuster-Lluch et al.[6] reported an incidence of glomerular hyperfiltration of 17.9% on admission to the ICU, with a mean CLCR of 142 mL/min/1.73 m2. Patients with an elevated GFR were primarily multi-trauma victims or postoperative patients, were younger, and had lower Acute Physiology and Chronic Health Evaluation (APACHE) II scores and higher urine outputs.
Brown et al.[3] also demonstrated elevated CLCR in a small cohort of young postoperative trauma patients, whose peak CLCR values reached 190 mL/min/1.73 m2. Albanese et al.[1] documented elevated CLCR in a subgroup of isolated traumatic brain-injured patients receiving norepinephrine. Significantly, clearances were elevated prior to institution of the vasopressor and remained elevated for the 24 hours of the study. A similar result was noted by Benmalek et al.[2] in their paper investigating the effects of dopamine in addition to norepinephrine in the management of post-traumatic intracranial hypertension. Recently, we demonstrated ARC in a cohort of patients with severe head injury receiving osmotherapy (hypertonic saline) and/or vasopressor infusion for the maintenance of cerebral perfusion pressure (unpublished data). The mean age of this group was 26 years, and 85% met the criteria for ARC during their ICU stay. Of note, clearances were elevated both on and off cerebral perfusion pressure therapy.
Shikuma et al.[83] have previously investigated the clearance of piperacillin in critically ill surgical patients with sepsis and normal renal function. In this relatively young cohort (mean age 44 years), wide interindividual variations were reported in key pharmacokinetic parameters, in addition to significantly elevated drug clearance. CLCR values and haemodynamic parameters were not provided, although a moderate correlation was reported between drug elimination and CLCR.[83] In contrast, Jacolot et al.[84] were unable to demonstrate any significant changes in the pharmacokinetics of cefpirome administered to traumatized patients with SIRS, as compared with matched healthy controls. Estimated CLCR values were elevated in the trauma group (median 147 mL/min), although this finding did not reach statistical significance. Of note, cefpirome was administered on average 9 days post-admission, no haemodynamic data were reported, and patients requiring vasopressor administration were excluded.[84]
It follows that younger patients (roughly <55 years), admitted post-trauma (particularly after head injury) or post-surgery appear to be at greatest risk of ARC. Higher antibacterial clearances have also been reported in patients with sepsis, haematological malignancy[25–28] and significant burn injury.[4,18–24,85]
1.5 Time Course of ARC
The time course of ARC in the critically ill is still uncertain, and some patients may develop this phenomenon later in their ICU admission. It is likely to vary between patients and depends on the pathophysiology of the presenting disease process and the type of clinical interventions undertaken. In the study by Fuster-Lluch et al.,[6] the authors observed that the prevalence of glomerular hyperfiltration (CLCR >120 mL/min/m2) was greatest on day 5 of the study. Brown et al.[3] reported peak CLCR levels on the fourth day, with levels returning to immediate postoperative values by day 7. In our recent observational study in traumatic brain injury, peak CLCR values were recorded after a mean of 4.7 days (range 0–11.5 days) of treatment (unpublished data). As the likely mechanisms involve a SIRS response, fluid loading and use of vasoactive medications,[83,86] ARC should always be considered in such a context. Where doubt exists, a timed urinary creatinine collection should be performed.
The clinical importance of ARC relates primarily to enhanced drug elimination, leading to subtherapeutic concentrations and potentially to treatment failure. Where drugs are administered to achieve a desired clinical effect (e.g. antihypertensives and sedatives), doses can be easily modified in the presence of ARC. However, for drugs with more subtle endpoints, such as antibacterials, and where the consequences of suboptimal therapy can be catastrophic, an estimate of drug clearance should be considered early to enable accurate dosing.
2. ARC as It Relates to Antibacterial Pharmacokinetics and Pharmacodynamics: Considerations for Dosing in the Intensive Care Unit
Antibacterials can be clearly classified on the basis of their bacterial kill characteristics. The β-lactam group of agents demonstrates time-dependent killing and, as such, the time for which the drug concentration remains above the minimum inhibitory concentration (MIC) for bacterial growth (T>MIC), is the best predictor of antibacterial efficacy.[87] Maintaining adequate plasma concentrations throughout the dosing interval is therefore essential, and the implications of ARC are most notable for β-lactams. In contrast, aminoglycosides have a concentration-dependent kill characteristic, whereby the effect is determined by the ratio of the maximum plasma drug concentration (Cmax) to the MIC (Cmax/MIC).[88] For other agents, such as fluoroquinolones and glycopeptides, the ratio of the area under the plasma concentration time curve from 0 to 24 hours (AUC24) to the MIC (AUC24/MIC) is the key pharmacokinetic-pharmacodynamic factor[89,90] associated with efficacy. Although more speculative, ARC could significantly influence the pharmacokinetic profile of renally eliminated agents in this group,[11] mandating more frequent dosing.
2.1 β-Lactam Antibacterials
The β-lactam group of antibacterials includes the penicillins, cephalosporins, carbapenems and monobactams, and is the group for which the greatest amount of data exist concerning the implications of ARC. In the absence of any significant post-antibacterial effect for a given agent, dosing schedules should aim to keep plasma concentrations above the MIC for 90–100% of the dosing interval.[91] In addition, concentrations 4–5 times the MIC are ideal, as bacterial killing is maximal.[92,93]
The majority of these agents are renally eliminated (through a mixture of glomerular filtration and tubular secretion), and a number of pharmacokinetic-pharmacodynamic papers have been published using different dosing regimens in the critically ill (table I). In addition, a correlation between CLCR and total drug clearance has been reported for a number of agents,[20,32,36,38–41,97,99,101,110,112,121,122] and recent work by Conil et al.[5] has highlighted the importance of CLCR as a key covariate in drug elimination, with a strong inverse relationship between CLCR and the minimum plasma drug concentration. Furthermore, both increased drug clearance and significant interindividual variability have been reported in the critically ill[34,40,83,95,99,109,119] (table I).
Although for some antibacterials the mean pharmacokinetic data reported for critically ill patients may not be greatly different from those in studies of healthy subjects (table I), the significant interindividual variability that is often documented indicates that summary statistics are not accurate in describing this phenomenon. In addition, as many critically ill patients manifest acute kidney injury and renal dysfunction, studies of small numbers in a heterogenous critically ill population will be underpowered to detect ARC. In those patients with normal renal function, ARC is likely to be common.[107,114]
A recent study by Noel et al.[123] involving the new, investigational, broad-spectrum cephalosporin ceftobiprole is worth consideration, as it highlights the potential clinical implications of ARC. Inferior cure rates, when compared with the combination of linezolid/ceftazidime, were documented in patients with ventilator-associated pneumonia (VAP), who were young (<45 years) or had an elevated CLCR at baseline (≥150 mL/min).[123] Although the clinical implications of this work are significant, it must be regarded primarily as hypothesis generating, as the study has yet to be published in a peer-reviewed journal, and no pharmacokinetic data have been provided.
2.1.1 Carbapenems
The carbapenems (meropenem, imipenem, panipenem, ertapenem, doripenem and biapenem) are considered a separate class of β-lactam antibacterials and also demonstrate time-dependent killing.[124] In vitro models have suggested that the carbapenem post-antibacterial effect enables these agents to require less T > MIC[125] for bacteriostatic activity (20%) and bactericidal activity (40%).[126] As demonstrated with other β-lactams, CLCR is a key covariate in predicting drug elimination,[36,37] which can be elevated in the critically ill (table I).[34,119] This in turn can lead to potentially subtherapeutic drug concentrations for large portions of the dosing interval.[34]
2.1.2 Implications of ARC for Dosing of β-Lactams
These data serve to underline the importance of ARC in dosing of β-lactam antibacterials in the critically ill and raises important questions as to the optimal strategy in this setting. Given the time-dependent kill characteristics of this class of antibacterials and the increased clearances documented in the critically ill, maintaining adequate drug concentrations through more frequent, extended or continuous dosing must be considered. Pharmacokinetic-pharmacodynamic data support administration by extended or continuous infusion,[32,103,127–133] and recent work has demonstrated improved clinical outcomes in subsets of critically ill patients, particularly VAP.[127,134–137] However, a recent systematic review of continuous dosing strategies has failed to demonstrate any clinical advantage,[138] although any role in the setting of ARC remains unknown. Despite the lack of conclusive outcome data, continuous infusion of β-lactams offers an attractive strategy to maintain therapeutic drug concentrations, and ongoing, prospective work in this area is needed to address the paucity of evidence.
2.2 Glycopeptide Antibacterials
The specific pharmacokinetic-pharmacodynamic profile of the glycopeptides is not fully understood, as some data suggest that these agents have time-dependent properties,[139–141] while animal studies have suggested that the Cmax/MIC ratio predicts efficacy against some micro-organisms.[142] Vancomycin is the most commonly prescribed glycopeptide, and recent studies have indicated that the AUC24/MIC ratio is the most important pharmacokinetic-pharmacodynamic parameter correlating with efficacy.[90,143] Clinical data have linked AUC24/MIC ratios of ≥400 with superior clinical and bacteriological responses in patients treated with vancomycin for meticillin-resistant Staphylococcus aureus infections of the lower respiratory tract.[144]
Vancomycin is primarily renally eliminated[145] through a mixture of glomerular filtration and renal tubular secretion. A large population pharmacokinetic study has demonstrated a significant correlation between vancomycin clearance and CLCR.[146] Additional work in the critically ill has confirmed this relationship[85,147,148] and suggests that CLCR accounts for >50% of the variability in vancomycin clearance in this population.[35] Furthermore, higher dose requirements have been demonstrated in patients concurrently receiving vasoactive medications,[145] in addition to augmented clearances in burns patients[85] and haematological malignancy.[27] Recently, Pea et al.[148] have developed dosing nomograms for vancomycin based on CLCR estimates, with good correlation between predicted and observed plasma concentrations.
Teicoplanin has a spectrum of activity similar to that of vancomycin, although its longer elimination half-life (in excess of 90 hours, due to high protein binding)[149] allows for once-daily dosing. A correlation between drug clearance and estimated CLCR of this agent has been reported in the critically ill,[28,33,150] and enhanced elimination has been demonstrated in the setting of hypoalbuminaemia[33] and severe neutropenia.[28]
2.2.1 Implications of ARC for Dosing of Glycopeptides
Despite a greater understanding of the relevant pharmacokinetic-pharmacodynamic properties of vancomycin, the optimal dosing regimen in the critically ill remains uncertain. Present levels of bacterial resistance suggest that trough concentrations of at least 15–20 mg/L are required, and in those displaying ARC, an increased frequency of dosing or continuous infusion may be appropriate. Using Monte Carlo simulations, previous studies have demonstrated that doses higher than those routinely prescribed in the ICU are needed to achieve the desired pharmacokinetic-pharmacodynamic targets, particularly with intermediate or drug-resistant strains.[35] As with β-lactams, continuous infusion has been proposed as a mechanism to achieve target steady-state concentrations. Although a large, prospective, multicentre study of intermittent dosing versus continuous infusion of glycopeptides failed to show any significant difference in microbiological or clinical outcomes,[151] Rello et al.[152] described a clinical benefit of continuous infusion in critically ill patients with VAP. As ARC significantly impacts on the elimination of vancomycin in subsets of critically ill patients, further research is urgently needed in this area.
Standard dosing strategies for teicoplanin in the critically ill employ a loading dose of 6 mg/kg 12-hourly for three doses,[150] followed by 6 mg/kg 24-hourly thereafter. However, recent work has recommended higher loading doses in hospitalized patients with sepsis,[153] likely as a result of an increased volume of distribution (Vd). In addition, doses of at least 12 mg/kg are required in endocarditis and in bone and joint infections,[154] and have also been advocated in VAP to achieve sufficient trough concentrations in lung tissue.[155]
2.3 Fluoroquinolone Antibacterials
This group of antibacterials includes ciprofloxacin, moxifloxacin, gatifloxacin and levofloxacin. Although a Cmax/MIC ratio >10 is critical for bacterial eradication,[156] Forrest et al.[89] have concluded that achieving an AUC24/MIC ratio >125 is associated with improved clinical outcomes in critically ill patients with Gram-negative infections. An AUC24/MIC ratio >100 also appears to prevent the emergence of bacterial resistance, particularly in the critically ill.[157]
Currently, there are limited data examining the impact of ARC on fluoroquinolone clearances in the critically ill, although it must be recognized that the effect may be limited, particularly as these agents have a large Vd. Data do exist, however, for ciprofloxacin[158,159] and levofloxacin[160] in this setting.
2.3.1 Implications of ARC for Dosing of Fluoroquinolones
The implications of ARC for dosing of this class of antibacterials is poorly understood. In patients with normal serum creatinine concentrations, we have previously shown that 8-hourly administration of ciprofloxacin was well tolerated and effective in severe sepsis,[161] although this regimen is still unlikely to reach the desired pharmacokinetic-pharmacodynamic targets.[162] Higher doses or alternative dosing regimens may be recommended in the future. In addition, there is a growing evidence base for higher doses of levofloxacin in the critically ill,[163] particularly as this agent is renally excreted. Further research is needed to address the paucity of knowledge in this area, given the apparent effect of ARC on some fluoroquinolones. Furthermore, suboptimal dosing of fluoroquinolones has been shown to promote the growth of drug-resistant mutants[126] and may be enhanced by rapid drug elimination.
2.4 Aminoglycoside Antibacterials
Aminoglycoside antibacterials demonstrate concentration-dependent killing, are excreted almost entirely unchanged by glomerular filtration[164] and demonstrate comparable, if not superior, clinical outcomes with single versus multiple daily dosing.[165,166] However, doses of 7 mg/kg confer a Cmax/MIC ratio of at least 10 (maximizing bacterial killing);[167] thus, in the setting of ARC, an increase in the dosing frequency to 18-hourly should be considered. This is particularly relevant in the critically ill, where investigators have demonstrated a significant impact of ICU interventions on pharmacokinetic parameters,[86] in addition to augmented drug clearances in sepsis,[168] haematological malignancy[169] and burns.[170] Recent work has confirmed that traditional dosing regimens are unlikely to meet the required pharmacokinetic-pharmacodynamic targets in the critically ill,[171] likely due to changes in the Vd in this population. As therapeutic drug monitoring is regularly employed in this setting, the impact of ARC can be clearly observed and dosing can be modified appropriately.
2.5 ARC Demonstrated with Other Antibacterials
ARC is likely to be relevant for any renally excreted antibacterial agent prescribed in the critically ill, particularly those agents that demonstrate time-dependent bacterial killing. Daptomycin is a novel lipopolypeptide antibacterial with good activity against most Gram-positive pathogens. Dose reduction has been recommended in patients with moderate to severe renal impairment,[172] as the primary route of elimination is via the kidneys. Currently, there are no data examining the impact of ARC on its prescription in the critically ill, although this may be of limited importance, as bacterial killing appears to be concentration dependent.[173] A lack of ARC was recently demonstrated in a study of febrile neutropenic patients compared with data from other patient populations.[174]
Linezolid is an oxazolidinone antibacterial with activity against multi-resistant Gram-positive pathogens. It is mostly hepatically metabolized before being renally cleared,[175] and dose modification is not currently recommended in patients with renal dysfunction.[176] However, previous data have confirmed time-dependent bacterial killing,[177,178] and a recent trial of alternate dosing strategies in the critically ill confirmed a pharmacokinetic advantage of continuous infusions in this population.[179] Increased total drug clearance was reported by the authors[179] and may represent augmented hepatic blood flow and/or function, in a manner similar to that of ARC. However conflicting data have been presented by others, suggesting that there is no difference in key tissue and plasma pharmacokinetic parameters in patients suffering from severe sepsis and septic shock compared with healthy subjects.[180]
3. Therapeutic Drug Monitoring
With increasing knowledge of the pharmacokinetic-pharmacodynamic properties of many antibacterials and the ability to measure plasma concentrations with relative precision, therapeutic drug monitoring (TDM) has a crucial role in optimizing antibacterial prescription in the critically ill. TDM is well established for aminoglycosides, where Cmax and trough concentration monitoring can be used to limit toxicity and improve efficacy.[181] In addition, trough concentrations can be used to guide vancomycin and teicoplanin prescriptions, in order to ensure adequate plasma concentrations.[29,182,183] However, outside these situations, TDM has played only a minor role in monitoring the adequacy of therapy with other routinely prescribed agents.
The significant pathophysiological changes encountered in the critically ill — and, in particular, the recognition of ARC — require the clinician to consider alternate dosing strategies for many agents. TDM represents a useful tool to enable accurate and timely dose modification to achieve the desired pharmacokinetic-pharmacodynamic targets and may significantly improve the clinical efficacy of antibacterial therapy in this population. In addition, the dynamic nature of critical illness and rapid changes in organ function mandate that dosing schedules be consistently evaluated, in order to reduce the likelihood of therapeutic failure or toxicity. As such, TDM should be regarded as an essential component of this process and must be readily available for a wide range of agents in the critical care environment.
4. Conclusions
Determining the optimal dosing regimen for any pharmaceutical is important but is of particular relevance for agents where the clinical response is difficult to assess. In addition, administering ‘the right dose’ is paramount where any delay in achieving therapeutic concentrations will result in increased morbidity and mortality. Such is the case with the prescription of antibacterials in the critically ill.
Accurate assessment of renal function in this setting is a complex task and usually focuses on identifying renal dysfunction, using regular estimation of serum creatinine concentrations. However, a growing literature base reinforces the hypothesis that ‘normal’ serum creatinine values may be associated with augmented clearances, particularly in young patients without pre-existing co-morbidity. Previous data have also identified several limitations in a number of commonly used equations to estimate the GFR in the critically ill. As such, a timed urinary creatinine collection remains the most accurate and routinely available method of assessing renal function in this population and should be employed routinely at the clinical level.
The likely mechanisms underlying this phenomenon involve an innate immune response characterized by SIRS and driven by endogenous mediator release. In the critically ill, this is further compounded by administration of intravenous fluids and vasoactive medications. Current evidence highlights young trauma patients as a population particularly ‘at risk’ although, to date, increasing drug doses in response to higher clearances has seldom been considered.
The implications in terms of enhanced drug elimination are significant, and subtherapeutic concentrations for lengthy periods of the dosing interval may predispose to treatment failure and/or emergence of resistant organisms. As this has largely been neglected in the clinical arena, more frequent estimations of CLCR and TDM are warranted to allow optimization of individual dosing requirements. Further research should now focus on identifying readily measurable predictors of ARC in the critically ill, validation of bedside tests to allow more frequent measurement and empirical adjustment of dosing regimens in this setting.
References
Albanese J, Leone M, Garnier F, et al. Renal effects of norepinephrine in septic and nonseptic patients. Chest 2004 Aug; 126(2): 534–9
Benmalek F, Behforouz N, Benoist JF, et al. Renal effects of low-dose do-pamine during vasopressor therapy for posttraumatic intracranial hypertension. Intensive Care Med 1999 Apr; 25(4): 399–405
Brown R, Babcock R, Talbert J, et al. Renal function in critically ill postoperative patients: sequential assessment of creatinine osmolar and free water clearance. Crit Care Med 1980 Feb; 8(2): 68–72
Conil JM, Georges B, Fourcade O, et al. Intermittent administration of ceftazidime to burns patients: influence of glomerular filtration. Int J Clin Pharmacol Ther 2007 Mar; 45(3): 133–42
Conil JM, Georges B, Mimoz O, et al. Influence of renal function on trough serum concentrations of piperacillin in intensive care unit patients. Intensive Care Med 2006 Dec; 32(12): 2063–6
Fuster-Lluch O, Geronimo-Pardo M, Peyro-Garcia R, et al. Glomerular hyperfiltration and albuminuria in critically ill patients. Anaesth Intensive Care 2008 Sep; 36(5): 674–80
Lipman J, Wallis SC, Boots RJ. Cefepime versus cefpirome: the importance of creatinine clearance. Anesth Analg 2003 Oct; 97(4): 1149–54
Loirat P, Rohan J, Baillet A, et al. Increased glomerular filtration rate in patients with major burns and its effect on the pharmacokinetics of tobramycin. N Engl J Med 1978 Oct 26; 299(17): 915–9
Sladen RN, Endo E, Harrison T. Two-hour versus 22-hour creatinine clearance in critically ill patients. Anesthesiology 1987 Dec; 67(6): 1013–6
Vincent F, El-Khoury N, Bonnard G, et al. Should a renal dose of norepinephrine stimulate hyperfiltration in head trauma patients?. Chest 2005 Jun; 127(6): 2282–3
Udy A, Roberts JA, Boots RJ, et al. You only find what you look for: the importance of high creatinine clearance in the critically ill. Anaesth Intensive Care 2009 Jan; 37(1): 11–3
Roberts JA, Kruger P, Paterson DL, et al. Antibiotic resistance: what’s dosing got to do with it?. Crit Care Med 2008 Aug; 36(8): 2433–40
Ibrahim EH, Sherman G, Ward S, et al. The influence of inadequate antimicrobial treatment of bloodstream infections on patient outcomes in the ICU setting. Chest 2000 Jul; 118(1): 146–55
Kollef MH, Sherman G, Ward S, et al. Inadequate antimicrobial treatment of infections: a risk factor for hospital mortality among critically ill patients. Chest 1999 Feb; 115(2): 462–74
Kumar A, Roberts D, Wood KE, et al. Duration of hypotension before initiation of effective antimicrobial therapy is the critical determinant of survival in human septic shock. Crit Care Med 2006 Jun; 34(6): 1589–96
MacArthur RD, Miller M, Albertson T, et al. Adequacy of early empiric antibiotic treatment and survival in severe sepsis: experience from the MONARCS Trial. Clin Infect Dis 2004 Jan 15; 38(2): 284–8
Finfer S, Bellomo R, Lipman J, et al. Adult-population incidence of severe sepsis in Australian and New Zealand intensive care units. Intensive Care Med 2004 Apr; 30(4): 589–96
Brater DC, Bawdon RE, Anderson SA, et al. Vancomycin elimination in patients with burn injury. Clin Pharmacol Ther 1986 Jun; 39(6): 631–4
Conil JM, Georges B, Lavit M, et al. A population pharmacokinetic approach to ceftazidime use in burn patients: influence of glomerular filtration, gender and mechanical ventilation. Br J Clin Pharmacol 2007 Jul; 64(1): 27–35
Conil JM, Georges B, Lavit M, et al. Pharmacokinetics of ceftazidime and cefepime in burn patients: the importance of age and creatinine clearance. Int J Clin Pharmacol Ther 2007 Oct; 45(10): 529–38
Friedrich LV, White RL, Kays MB, et al. Aztreonam pharmacokinetics in burn patients. Antimicrob Agents Chemother 1991 Jan; 35(1): 57–61
Garrelts JC, Jost G, Kowalsky SF, et al. Ciprofloxacin pharmacokinetics in burn patients. Antimicrob Agents Chemother 1996 May; 40(5): 1153–6
Garrelts JC, Peterie JD. Altered vancomycin dose vs serum concentration relationship in burn patients. Clin Pharmacol Ther 1988 Jul; 44(1): 9–13
Rybak MJ, Albrecht LM, Berman JR, et al. Vancomycin pharmacokinetics in burn patients and intravenous drug abusers. Antimicrob Agents Chemother 1990 May; 34(5): 792–5
Lamoth F, Buclin T, Csajka C, et al. Reassessment of recommended imipenem doses in febrile neutropenic patients with hematological malignancies. Antimicrob Agents Chemother 2009 Feb; 53(2): 785–7
Pea F, Viale P, Candoni A, et al. Teicoplanin in patients with acute leukaemia and febrile neutropenia: a special population benefiting from higher dosages. Clin Pharmacokinet 2004; 43(6): 405–15
Fernandez de Gatta MM, Fruns I, Hernandez JM, et al. Vancomycin pharmacokinetics and dosage requirements in hematologic malignancies. Clin Pharm 1993 Jul; 12(7): 515–20
Lortholary O, Tod M, Rizzo N, et al. Population pharmacokinetic study of teicoplanin in severely neutropenic patients. Antimicrob Agents Chemother 1996 May; 40(5): 1242–7
Roberts JA, Lipman J. Antibacterial dosing in intensive care: pharmacokinetics, degree of disease and pharmacodynamics of sepsis. Clin Pharmacokinet 2006; 45(8): 755–73
Stevens LA, Coresh J, Greene T, et al. Assessing kidney function: measured and estimated glomerular filtration rate. N Engl J Med 2006 Jun 8; 354(23): 2473–83
Sunder-Plassmann G, Horl WH. A critical appraisal for definition of hyperfiltration [letter]. Am J Kidney Dis 2004 Feb; 43(2): 396; author reply 397
Angus BJ, Smith MD, Suputtamongkol Y, et al. Pharmacokinetic-pharmacodynamic evaluation of ceftazidime continuous infusion vs intermittent bolus injection in septicaemic melioidosis. Br J Clin Pharmacol 2000 Aug; 50(2): 184–91
Barbot A, Venisse N, Rayeh F, et al. Pharmacokinetics and pharmacodynamics of sequential intravenous and subcutaneous teicoplanin in critically ill patients without vasopressors. Intensive Care Med 2003 Sep; 29(9): 1528–34
Burkhardt O, Kumar V, Katterwe D, et al. Ertapenem in critically ill patients with early-onset ventilator-associated pneumonia: pharmacokinetics with special consideration of free-drug concentration. J Antimicrob Chemother 2007 Feb; 59(2): 277–84
del Mar Fernandez de Gatta Garcia M, Revilla N, Calvo MV, et al. Pharmacokinetic/pharmacodynamic analysis of vancomycin in ICU patients. Intensive Care Med 2007 Feb; 33(2): 279–85
Ikawa K, Morikawa N, Ikeda K, et al. Pharmacokinetic-pharmacodynamic target attainment analysis of biapenem in adult patients: a dosing strategy. Chemotherapy 2008; 54(5): 386–94
Ikawa K, Morikawa N, Uehara S, et al. Pharmacokinetic-pharmacodynamic target attainment analysis of doripenem in infected patients. Int J Antimicrob Agents 2009 Mar; 33(3): 276–9
Li C, Kuti JL, Nightingale CH, et al. Population pharmacokinetics and pharmacodynamics of piperacillin/tazobactam in patients with complicated intraabdominal infection. J Antimicrob Chemother 2005 Aug; 56(2): 388–95
Lipman J, Wallis SC, Rickard C. Low plasma cefepime levels in critically ill septic patients: pharmacokinetic modeling indicates improved troughs with revised dosing. Antimicrob Agents Chemother 1999 Oct; 43(10): 2559–61
Lipman J, Wallis SC, Rickard CM, et al. Low cefpirome levels during twice daily dosing in critically ill septic patients: pharmacokinetic modelling calls for more frequent dosing. Intensive Care Med 2001 Feb; 27(2): 363–70
Young RJ, Lipman J, Gin T, et al. Intermittent bolus dosing of ceftazidime in critically ill patients. J Antimicrob Chemother 1997 Aug; 40(2): 269–73
Bone RC, Balk RA, Cerra FB, et al. Definitions for sepsis and organ failure and guidelines for the use of innovative therapies in sepsis. The ACCP/SCCM Consensus Conference Committee, American College of Chest Physicians/Society of Critical Care Medicine. Chest 1992 Jun; 101(6): 1644–55
Kohl BA, Deutschman CS. The inflammatory response to surgery and trauma. Curr Opin Crit Care 2006 Aug; 12(4): 325–32
Ishikawa M, Nishioka M, Hanaki N, et al. Postoperative metabolic and circulatory responses in patients that express SIRS after major digestive surgery. Hepatogastroenterology 2006 Mar–Apr; 53(68): 228–33
Levy MM, Fink MP, Marshall JC, et al. 2001 SCCM/ESICM/ACCP/ATS/SIS International Sepsis Definitions Conference. Intensive Care Med 2003 Apr; 29(4): 530–8
Parrillo JE, Parker MM, Natanson C, et al. Septic shock in humans: advances in the understanding of pathogenesis, cardiovascular dysfunction, and therapy. Ann Intern Med 1990 Aug 1; 113(3): 227–42
Di Giantomasso D, May CN, Bellomo R. Vital organ blood flow during hyperdynamic sepsis. Chest 2003 Sep; 124(3): 1053–9
Dellinger RP, Levy MM, Carlet JM, et al. Surviving Sepsis Campaign: international guidelines for management of severe sepsis and septic shock: 2008. Crit Care Med 2008 Jan; 36(1): 296–327
Rivers E, Nguyen B, Havstad S, et al. Early goal-directed therapy in the treatment of severe sepsis and septic shock. N Engl J Med 2001 Nov 8; 345(19): 1368–77
Wan L, Bellomo R, May CN. The effects of normal and hypertonic saline on regional blood flow and oxygen delivery. Anesth Analg 2007 Jul; 105(1): 141–7
Bellomo R, Wan L, May C. Vasoactive drugs and acute kidney injury. Crit Care Med 2008 Apr; 36(4 Suppl.): S179–86
Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow. Intensive Care Med 2002 Dec; 28(12): 1804–9
Di Giantomasso D, May CN, Bellomo R. Norepinephrine and vital organ blood flow during experimental hyperdynamic sepsis. Intensive Care Med 2003 Oct; 29(10): 1774–81
Bellomo R, Kellum JA, Wisniewski SR, et al. Effects of norepinephrine on the renal vasculature in normal and endotoxemic dogs. Am J Respir Crit Care Med 1999 Apr; 159(4 Pt 1): 1186–92
Desjars P, Pinaud M, Bugnon D, et al. Norepinephrine therapy has no deleterious renal effects in human septic shock. Crit Care Med 1989 May; 17(5): 426–9
Marin C, Eon B, Saux P, et al. Renal effects of norepinephrine used to treat septic shock patients. Crit Care Med 1990 Mar; 18(3): 282–5
Redl-Wenzl EM, Armbruster C, Edelmann G, et al. The effects of norepinephrine on hemodynamics and renal function in severe septic shock states. Intensive Care Med 1993; 19(3): 151–4
Wan L, Bagshaw SM, Langenberg C, et al. Pathophysiology of septic acute kidney injury: what do we really know?. Crit Care Med 2008 Apr; 36 (4 Suppl.): S198–203
Bagshaw SM, Uchino S, Bellomo R, et al. Septic acute kidney injury in critically ill patients: clinical characteristics and outcomes. Clin J Am Soc Nephrol 2007 May; 2(3): 431–9
Hoste EA, Damen J, Vanholder RC, et al. Assessment of renal function in recently admitted critically ill patients with normal serum creatinine. Nephrol Dial Transplant 2005 Apr; 20(4): 747–53
Jones CA, McQuillan GM, Kusek JW, et al. Serum creatinine levels in the US population: third National Health and Nutrition Examination Survey. Am J Kidney Dis 1998 Dec; 32(6): 992–9
Cartin-Ceba R, Afessa B, Gajic O. Low baseline serum creatinine concentration predicts mortality in critically ill patients independent of body mass index. Crit Care Med 2007 Oct; 35(10): 2420–3
Levey AS, Bosch JP, Lewis JB, et al. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med 1999 Mar 16; 130(6): 461–70
Lin J, Knight EL, Hogan ML, et al. A comparison of prediction equations for estimating glomerular filtration rate in adults without kidney disease. J Am Soc Nephrol 2003 Oct; 14(10): 2573–80
Poggio ED, Wang X, Greene T, et al. Performance of the modification of diet in renal disease and Cockcroft-Gault equations in the estimation of GFR in health and in chronic kidney disease. J Am Soc Nephrol 2005 Feb; 16(2): 459–66
Rule AD, Larson TS, Bergstralh EJ, et al. Using serum creatinine to estimate glomerular filtration rate: accuracy in good health and in chronic kidney disease. Ann Intern Med 2004 Dec 21; 141(12): 929–37
Cockcroft DW, Gault MH. Prediction of creatinine clearance from serum creatinine. Nephron 1976; 16(1): 31–41
Conil JM, Georges B, Fourcade O, et al. Assessment of renal function in clinical practice at the bedside of burn patients. Br J Clin Pharmacol 2007 May; 63(5): 583–94
Snider RD, Kruse JA, Bander JJ, et al. Accuracy of estimated creatinine clearance in obese patients with stable renal function in the intensive care unit. Pharmacotherapy 1995 Nov-Dec; 15(6): 747–53
Poggio ED, Nef PC, Wang X, et al. Performance of the Cockcroft-Gault and modification of diet in renal disease equations in estimating GFR in ill hospitalized patients. Am J Kidney Dis 2005 Aug; 46(2): 242–52
Jelliffe R. Estimation of creatinine clearance in patients with unstable renal function, without a urine specimen. Am J Nephrol 2002 Jul–Aug; 22(4): 320–4
Herrera-Gutierrez ME, Seller-Perez G, Banderas-Bravo E, et al. Replacement of 24-h creatinine clearance by 2-h creatinine clearance in intensive care unit patients: a single-center study. Intensive Care Med 2007 Nov; 33(11): 1900–6
Pong S, Seto W, Abdolell M, et al. 12-hour versus 24-hour creatinine clearance in critically ill pediatric patients. Pediatr Res 2005 Jul; 58(1): 83–8
Wells M, Lipman J. Measurements of glomerular filtration in the intensive care unit are only a rough guide to renal function. S Afr J Surg 1997 Feb; 35(1): 20–3
Wells M, Lipman J. Pitfalls in the prediction of renal function in the intensive care unit: a review. S Afr J Surg 1997 Feb; 35(1): 16–9
Bonate PL, Reith K, Weir S. Drug interactions at the renal level: implications for drug development. Clin Pharmacokinet 1998 May; 34(5): 375–404
Bjornsson TD. Use of serum creatinine concentrations to determine renal function. Clin Pharmacokinet 1979 May–Jun; 4(3): 200–22
Orlando R, Floreani M, Padrini R, et al. Determination of inulin clearance by bolus intravenous injection in healthy subjects and ascitic patients: equivalence of systemic and renal clearances as glomerular filtration markers. Br J Clin Pharmacol 1998 Dec; 46(6): 605–9
Buclin T, Pechere-Bertschi A, Sechaud R, et al. Sinistrin clearance for determination of glomerular filtration rate: a reappraisal of various approaches using a new analytical method. J Clin Pharmacol 1997 Aug; 37(8): 679–92
Parikh CR, Devarajan P. New biomarkers of acute kidney injury. Crit Care Med 2008 Apr; 36 (4 Suppl.): S159–65
Gross AS, McLachlan AJ, Minns I, et al. Simultaneous administration of a cocktail of markers to measure renal drug elimination pathways: absence of a pharmacokinetic interaction between fluconazole and sinistrin, p-aminohippuric acid and pindolol. Br J Clin Pharmacol 2001 Jun; 51(6): 547–55
McLachlan AJ, Gross AS, Beal JL, et al. Analytical validation for a series of marker compounds used to assess renal drug elimination processes. Ther Drug Monit 2001 Feb; 23(1): 39–46
Shikuma LR, Ackerman BH, Weaver RH, et al. Effects of treatment and the metabolic response to injury on drug clearance: a prospective study with piperacillin. Crit Care Med 1990 Jan; 18(1): 37–41
Jacolot A, Incagnoli P, Edouard AR, et al. Pharmacokinetics of cefpirome during the posttraumatic systemic inflammatory response syndrome. Intensive Care Med 1999 May; 25(5): 486–91
Dailly E, Le Floch R, Deslandes G, et al. Influence of glomerular filtration rate on the clearance of vancomycin administered by continuous infusion in burn patients. Int J Antimicrob Agents 2008 Jun; 31(6): 537–9
Lugo G, Castaneda-Hernandez G. Relationship between hemodynamic and vital support measures and pharmacokinetic variability of amikacin in critically ill patients with sepsis. Crit Care Med 1997 May; 25(5): 806–11
Craig WA. Pharmacokinetic/pharmacodynamic parameters: rationale for antibacterial dosing of mice and men. Clin Infect Dis 1998 Jan; 26(1): 1–10; quiz 11–2
Moore RD, Lietman PS, Smith CR. Clinical response to aminoglycoside therapy: importance of the ratio of peak concentration to minimal inhibitory concentration. J Infect Dis 1987 Jan; 155(1): 93–9
Forrest A, Nix DE, Ballow CH, et al. Pharmacodynamics of intravenous ciprofloxacin in seriously ill patients. Antimicrob Agents Chemother 1993 May; 37(5): 1073–81
Rybak MJ. The pharmacokinetic and pharmacodynamic properties of vancomycin. Clin Infect Dis 2006 Jan 1; 42 Suppl. 1: S35–9
Turnidge JD. The pharmacodynamics of beta-lactams. Clin Infect Dis 1998 Jul; 27(1): 10–22
Vogelman B, Craig WA. Kinetics of antimicrobial activity. J Pediatr 1986 May; 108(5 Pt 2): 835–40
Vogelman BS, Craig WA. Postantibiotic effects. J Antimicrob Chemother 1985 Jan; 15 Suppl. A: 37–46
Barbhaiya RH, Forgue ST, Gleason CR, et al. Pharmacokinetics of cefepime after single and multiple intravenous administrations in healthy subjects. Antimicrob Agents Chemother 1992 Mar; 36(3): 552–7
Georges B, Conil JM, Seguin T, et al. Cefepime in intensive care unit patients: validation of a population pharmacokinetic approach and influence of covariables. Int J Clin Pharmacol Ther 2008 Apr; 46(4): 157–64
Kieft H, Hoepelman AI, Knupp CA, et al. Pharmacokinetics of cefepime in patients with the sepsis syndrome. J Antimicrob Chemother 1993 Nov; 32 Suppl. B: 117–22
Joukhadar C, Klein N, Mayer BX, et al. Plasma and tissue pharmacokinetics of cefpirome in patients with sepsis. Crit Care Med 2002 Jul; 30(7): 1478–82
Patel IH, Chen S, Parsonnet M, et al. Pharmacokinetics of ceftriaxone in humans. Antimicrob Agents Chemother 1981 Nov; 20(5): 634–41
Joynt GM, Lipman J, Gomersall CD, et al. The pharmacokinetics of oncedaily dosing of ceftriaxone in critically ill patients. J Antimicrob Chemother 2001 Apr; 47(4): 421–9
Heinemeyer G, Link J, Weber W, et al. Clearance of ceftriaxone in critical care patients with acute renal failure. Intensive Care Med 1990; 16(7): 448–53
Van Dalen R, Vree T, Baars IM. Influence of protein binding and severity of illness on renal elimination of four cephalosporin drugs in intensive-care patients. Pharm Weekbl Sci 1987 Apr; 9(2): 98–103
Sommers DK, Walters L, Van Wyk M, et al. Pharmacokinetics of ceftazidime in male and female volunteers. Antimicrob Agents Chemother 1983 Jun; 23(6): 892–6
Hanes SD, Wood GC, Herring V, et al. Intermittent and continuous ceftazidime infusion for critically ill trauma patients. Am J Surg 2000 Jun; 179(6): 436–40
Gomez CM, Cordingly JJ, Palazzo MG. Altered pharmacokinetics of ceftazidime in critically ill patients. Antimicrob Agents Chemother 1999 Jul; 43(7): 1798–802
Buijk SL, Gyssens IC, Mouton JW, et al. Pharmacokinetics of ceftazidime in serum and peritoneal exudate during continuous versus intermittent administration to patients with severe intra-abdominal infections. J Antimicrob Chemother 2002 Jan; 49(1): 121–8
Batra VK, Morrison JA, Lasseter KC, et al. Piperacillin kinetics. Clin Pharmacol Ther 1979 Jul; 26(1): 41–53
Roberts JA, Roberts MS, Robertson TA, et al. Piperacillin penetration into tissue of critically ill patients with sepsis: bolus versus continuous administration?. Crit Care Med 2009 Mar; 37(3): 926–33
Bax RP, Bastain W, Featherstone A, et al. The pharmacokinetics of meropenem in volunteers. J Antimicrob Chemother 1989 Sep; 24 Suppl. A: 311–20
Novelli A, Adembri C, Livi P, et al. Pharmacokinetic evaluation of meropenem and imipenem in critically ill patients with sepsis. Clin Pharmacokinet 2005; 44(5): 539–49
Kitzes-Cohen R, Farin D, Piva G, et al. Pharmacokinetics and pharmacodynamics of meropenem in critically ill patients. Int J Antimicrob Agents 2002 Feb; 19(2): 105–10
Karjagin J, Lefeuvre S, Oselin K, et al. Pharmacokinetics of meropenem determined by microdialysis in the peritoneal fluid of patients with severe peritonitis associated with septic shock. Clin Pharmacol Ther 2008 Mar; 83(3): 452–9
Lovering AM, Vickery CJ, Watkin DS, et al. The pharmacokinetics of meropenem in surgical patients with moderate or severe infections. J Antimicrob Chemother 1995 Jul; 36(1): 165–72
Thalhammer F, Traunmuller F, El Menyawi I, et al. Continuous infusion versus intermittent administration of meropenem in critically ill patients. J Antimicrob Chemother 1999 Apr; 43(4): 523–7
Roberts JA, Kirkpatrick CM, Roberts MS, et al. Meropenem dosing in critically ill patients with sepsis and without renal dysfunction intermittent bolus versus continuous administration?. Monte Carlo dosing simulations and subcutaneous tissue distribution. J Antimicrob Chemother 2009 Jul; 64(1): 142–50
Norrby SR, Bjornegard B, Ferber F, et al. Pharmacokinetics of imipenem in healthy volunteers. J Antimicrob Chemother 1983 Dec; 12 Suppl. D: 109–24
Tegeder I, Schmidtko A, Brautigam L, et al. Tissue distribution of imipenem in critically ill patients. Clin Pharmacol Ther 2002 May; 71(5): 325–33
Sakka SG, Glauner AK, Bulitta JB, et al. Population pharmacokinetics and pharmacodynamics of continuous versus short-term infusion of imipenem-cilastatin in critically ill patients in a randomized, controlled trial. Antimicrob Agents Chemother 2007 Sep; 51(9): 3304–10
Majumdar AK, Musson DG, Birk KL, et al. Pharmacokinetics of ertapenem in healthy young volunteers. Antimicrob Agents Chemother 2002 Nov; 46(11): 3506–11
Brink AJ, Richards GA, Schillack V, et al. Pharmacokinetics of once-daily dosing of ertapenem in critically ill patients with severe sepsis. Int J Antimicrob Agents 2009 May; 33(5): 432–6
Boselli E, Breilh D, Saux MC, et al. Pharmacokinetics and lung concentrations of ertapenem in patients with ventilator-associated pneumonia. Intensive Care Med 2006 Dec; 32(12): 2059–62
Roos JF, Lipman J, Kirkpatrick CM. Population pharmacokinetics and pharmacodynamics of cefpirome in critically ill patients against Gramnegative bacteria. Intensive Care Med 2007 May; 33(5): 781–8
Tam VH, McKinnon PS, Akins RL, et al. Pharmacokinetics and pharmacodynamics of cefepime in patients with various degrees of renal function. Antimicrob Agents Chemother 2003 Jun; 47(6): 1853–61
Noel G, Strauss R, Shah A, et al. Ceftobiprole versus ceftazidime combined with linezolid for treatment of patients with nosocomial pneumonia [poster no. K-486]. 48th Annual Interscience Conference on Antimicrobial Agents and Chemotherapy/46th Infectious Diseases Society of America Annual Meeting; 2008 Oct 25–28; Washington, DC
Mouton JW, Touzw DJ, Horrevorts AM, et al. Comparative pharmacokinetics of the carbapenems: clinical implications. Clin Pharmacokinet 2000 Sep;39(3): 185–201
Bustamante CI, Drusano GL, Tatem BA, et al. Postantibiotic effect of imipenem on Pseudomonas aeruginosa. Antimicrob Agents Chemother 1984 Nov; 26(5): 678–82
Drusano GL. Prevention of resistance: a goal for dose selection for antimicrobial agents. Clin Infect Dis 2003 Jan 15; 36 Suppl. 1: S42–50
Roberts JA, Boots R, Rickard CM, et al. Is continuous infusion ceftriaxone better than once-a-day dosing in intensive care?. A randomized controlled pilot study. J Antimicrob Chemother 2007 Feb; 59(2): 285–91
Lipman J, Gomersall CD, Gin T, et al. Continuous infusion ceftazidime in intensive care: a randomized controlled trial. J Antimicrob Chemother 1999 Feb; 43(2): 309–11
Georges B, Conil JM, Cougot P, et al. Cefepime in critically ill patients: continuous infusion vs an intermittent dosing regimen. Int J Clin Pharmacol Ther 2005 Aug; 43(8): 360–9
McNabb JJ, Nightingale CH, Quintiliani R, et al. Cost-effectiveness of ceftazidime by continuous infusion versus intermittent infusion for nosocomial pneumonia. Pharmacotherapy 2001 May; 21(5): 549–55
Mouton JW, Vinks AA, Punt NC. Pharmacokinetic-pharmacodynamic modeling of activity of ceftazidime during continuous and intermittent infusion. Antimicrob Agents Chemother 1997 Apr; 41(4): 733–8
Nicolau DP, McNabb J, Lacy MK, et al. Continuous versus intermittent administration of ceftazidime in intensive care unit patients with nosocomial pneumonia. Int J Antimicrob Agents 2001 Jun; 17(6): 497–504
Roberts JA, Paratz J, Paratz E, et al. Continuous infusion of beta-lactam antibiotics in severe infections: a review of its role. Int J Antimicrob Agents 2007 Jul; 30(1): 11–8
Lodise Jr TP, Lomaestro B, Drusano GL. Piperacillin-tazobactam for Pseudomonas aeruginosa infection: clinical implications of an extended-infusion dosing strategy. Clin Infect Dis 2007 Feb 1; 44(3): 357–63
Lorente L, Lorenzo L, Martin MM, et al. Meropenem by continuous versus intermittent infusion in ventilator-associated pneumonia due to Gramnegative bacilli. Ann Pharmacother 2006 Feb; 40(2): 219–23
Lorente L, Jimenez A, Martin MM, et al. Clinical cure of ventilator-associated pneumonia treated with piperacillin/tazobactam administered by continuous or intermittent infusion. Int J Antimicrob Agents 2009 May; 33(5): 464–8
Lorente L, Jimenez A, Palmero S, et al. Comparison of clinical cure rates in adults with ventilator-associated pneumonia treated with intravenous ceftazidime administered by continuous or intermittent infusion: a retrospective, nonrandomized, open-label, historical chart review. Clin Ther 2007 Nov; 29(11): 2433–9
Roberts JA, Webb SA, Paterson DL, et al. A systematic review on clinical benefits of continuous administration of beta-lactam antibiotics. Crit Care Med 2009 Jun; 37(6): 2071–8
Chambers HF, Kennedy S. Effects of dosage, peak and trough concentrations in serum, protein binding, and bactericidal rate on efficacy of teicoplanin in a rabbit model of endocarditis. Antimicrob Agents Chemother 1990 Apr; 34(4): 510–4
Larsson AJ, Walker KJ, Raddatz JK, et al. The concentration-independent effect of monoexponential and biexponential decay in vancomycin con-centrations on the killing of Staphylococcus aureus under aerobic and anaerobic conditions. J Antimicrob Chemother 1996 Oct; 38(4): 589–97
Lowdin E, Odenholt I, Cars O. In vitro studies of pharmacodynamic properties of vancomycin against Staphylococcus aureus and Staphylococcus epidermidis. Antimicrob Agents Chemother 1998 Oct; 42(10): 2739–44
Knudsen JD, Fuursted K, Raber S, et al. Pharmacodynamics of glycopeptides in the mouse peritonitis model of Streptococcus pneumoniae or Staphylococcus aureus infection. Antimicrob Agents Chemother 2000 May; 44(5): 1247–54
Craig WA. Basic pharmacodynamics of antibacterials with clinical applications to the use of beta-lactams, glycopeptides, and linezolid. Infect Dis Clin North Am 2003 Sep; 17(3): 479–501
Moise-Broder PA, Forrest A, Birmingham MC, et al. Pharmacodynamics of vancomycin and other antimicrobials in patients with Staphylococcus aureus lower respiratory tract infections. Clin Pharmacokinet 2004; 43(13): 925–42
Pea F, Porreca L, Baraldo M, et al. High vancomycin dosage regimens required by intensive care unit patients cotreated with drugs to improve haemodynamics following cardiac surgical procedures. J Antimicrob Chemother 2000 Mar; 45(3): 329–35
Ducharme MP, Slaughter RL, Edwards DJ. Vancomycin pharmacokinetics in a patient population: effect of age, gender, and body weight. Ther Drug Monit 1994 Oct; 16(5): 513–8
Llopis-Salvia P, Jimenez-Torres NV. Population pharmacokinetic parameters of vancomycin in critically ill patients. J Clin Pharm Ther 2006 Oct; 31(5): 447–54
Pea F, Furlanut M, Negri C, et al. Prospectively validated dosing nomograms for maximizing the pharmacodynamics of vancomycin administered by continuous infusion in the critically ill patients. Antimicrob Agents Chemother 2009 May; 53(5): 1863–7
Del Favero A, Patoia L, Rosina R, et al. Pharmacokinetics and tolerability of teicoplanin in healthy volunteers after single increasing doses. Antimicrob Agents Chemother 1991 Dec; 35(12): 2551–7
Pea F, Brollo L, Viale P, et al. Teicoplanin therapeutic drug monitoring in critically ill patients: a retrospective study emphasizing the importance of a loading dose. J Antimicrob Chemother 2003 Apr; 51(4): 971–5
Wysocki M, Delatour F, Faurisson F, et al. Continuous versus intermittent infusion of vancomycin in severe staphylococcal infections: prospective multicenter randomized study. Antimicrob Agents Chemother 2001 Sep; 45(9): 2460–7
Rello J, Sole-Violan J, Sa-Borges M, et al. Pneumonia caused by oxacillinresistant Staphylococcus aureus treated with glycopeptides. Crit Care Med 2005 Sep; 33(9): 1983–7
Brink AJ, Richards GA, Cummins RR, et al. Recommendations to achieve rapid therapeutic teicoplanin plasma concentrations in adult hospitalised patients treated for sepsis. Int J Antimicrob Agents 2008 Nov; 32(5): 455–8
Wilson AP. Clinical pharmacokinetics of teicoplanin. Clin Pharmacokinet 2000 Sep; 39(3): 167–83
Mimoz O, Rolland D, Adoun M, et al. Steady-state trough serum and epithelial lining fluid concentrations of teicoplanin 12mg/kg per day in patients with ventilator-associated pneumonia. Intensive Care Med 2006 May; 32(5): 775–9
Drusano GL, Johnson DE, Rosen M, et al. Pharmacodynamics of a fluoroquinolone antimicrobial agent in a neutropenic rat model of Pseudomonas sepsis. Antimicrob Agents Chemother 1993 Mar; 37(3): 483–90
Thomas JK, Forrest A, Bhavnani SM, et al. Pharmacodynamic evaluation of factors associated with the development of bacterial resistance in acutely ill patients during therapy. Antimicrob Agents Chemother 1998 Mar; 42(3): 521–7
Conil JM, Georges B, de Lussy A, et al. Ciprofloxacin use in critically ill patients: pharmacokinetic and pharmacodynamic approaches. Int J Antimicrob Agents 2008 Dec; 32(6): 505–10
van Zanten AR, Polderman KH, van Geijlswijk IM, et al. Ciprofloxacin pharmacokinetics in critically ill patients: a prospective cohort study. J Crit Care 2008 Sep; 23(3): 422–30
Pea F, Di Qual E, Cusenza A, et al. Pharmacokinetics and pharmacodynamics of intravenous levofloxacin in patients with early-onset ventilator-associated pneumonia. Clin Pharmacokinet 2003; 42(6): 589–98
Lipman J, Scribante J, Gous AG, et al. Pharmacokinetic profiles of high-dose intravenous ciprofloxacin in severe sepsis. The Baragwanath Ciprofloxacin Study Group. Antimicrob Agents Chemother 1998 Sep; 42(9): 2235–9
Sun HK, Kuti JL, Nicolau DP. Pharmacodynamics of antimicrobials for the empirical treatment of nosocomial pneumonia: a report from the OPTAMA Program. Crit Care Med 2005 Oct; 33(10): 2222–7
Graninger W, Zeitlinger M. Clinical applications of levofloxacin for severe infections. Chemotherapy 2004; 50 Suppl. 1: 16–21
Chambers HF, Sande MA. Antimicrobial agents: the aminoglycosides. In: Goodman LS, Limberd LE, Milinoff PB, et al., editors. Goodman and Gilman’s: the pharmacological basis of therapeutics. 9th ed. New York: McGraw Hill, 1996: 1103–21
Olsen KM, Rudis MI, Rebuck JA, et al. Effect of once-daily dosing vs multiple daily dosing of tobramycin on enzyme markers of nephrotoxicity. Crit Care Med 2004 Aug; 32(8): 1678–82
Prins JM, Buller HR, Kuijper EJ, et al. Once versus thrice daily gentamicin in patients with serious infections. Lancet 1993 Feb 6; 341(8841): 335–9
Kashuba AD, Nafziger AN, Drusano GL, et al. Optimizing aminoglycoside therapy for nosocomial pneumonia caused by Gram-negative bacteria. Antimicrob Agents Chemother 1999 Mar; 43(3): 623–9
Beckhouse MJ, Whyte IM, Byth PL, et al. Altered aminoglycoside pharmacokinetics in the critically ill. Anaesth Intensive Care 1988 Nov; 16(4): 418–22
Romano S, Fdez de Gatta MM, Calvo MV, et al. Population pharmacokinetics of amikacin in patients with haematological malignancies. J Antimicrob Chemother 1999 Aug; 44(2): 235–42
Conil JM, Georges B, Breden A, et al. Increased amikacin dosage requirements in burn patients receiving a once-daily regimen. Int J Antimicrob Agents 2006 Sep; 28(3): 226–30
Rea RS, Capitano B, Bies R, et al. Suboptimal aminoglycoside dosing in critically ill patients. Ther Drug Monit 2008 Dec; 30(6): 674–81
Schriever CA, Fernandez C, Rodvold KA, et al. Daptomycin: a novel cyclic lipopeptide antimicrobial. Am J Health Syst Pharm 2005 Jun 1; 62(11): 1145–58
Safdar N, Andes D, Craig WA. In vivo pharmacodynamic activity of daptomycin. Antimicrob Agents Chemother 2004 Jan; 48(1): 63–8
Bubalo JS, Munar MY, Cherala G, et al. Daptomycin pharmacokinetics in adult oncology patients with neutropenic fever. Antimicrob Agents Chemother 2009 Feb; 53(2): 428–34
MacGowan AP. Pharmacokinetic and pharmacodynamic profile of linezolid in healthy volunteers and patients with Gram-positive infections. J Antimicrob Chemother 2003 May; 51 Suppl. 2: ii17–25
Brier ME, Stalker DJ, Aronoff GR, et al. Pharmacokinetics of linezolid in subjects with renal dysfunction. Antimicrob Agents Chemother 2003 Sep; 47(9): 2775–80
Andes D, van Ogtrop ML, Peng J, et al. In vivo pharmacodynamics of a new oxazolidinone (linezolid). Antimicrob Agents Chemother 2002 Nov; 46(11): 3484–9
Gentry-Nielsen MJ, Olsen KM, Preheim LC. Pharmacodynamic activity and efficacy of linezolid in a ratmodel of pneumococcal pneumonia. Antimicrob Agents Chemother 2002 May; 46(5): 1345–51
Adembri C, Fallani S, Cassetta MI, et al. Linezolid pharmacokinetic/pharmacodynamic profile in critically ill septic patients: intermittent versus continuous infusion. Int J Antimicrob Agents 2008 Feb; 31(2): 122–9
Thallinger C, Buerger C, Plock N, et al. Effect of severity of sepsis on tissue concentrations of linezolid. J Antimicrob Chemother 2008 Jan; 61(1): 173–6
Nicolau DP, Freeman CD, Belliveau PP, et al. Experience with a once-daily aminoglycoside program administered to 2,184 adult patients. Antimicrob Agents Chemother 1995 Mar; 39(3): 650–5
MacGowan AP. Pharmacodynamics pharmacokinetics, and therapeutic drug monitoring of glycopeptides. Ther Drug Monit 1998 Oct; 20(5): 473–7
Roberts JA, Lipman J. Pharmacokinetic issues for antibiotics in the critically ill patient. Crit Care Med 2009 Mar; 37(3): 840–51; quiz 859
Acknowledgements
This research was supported, in part, by NHMRC Project Grant no. 519702 from the Australian National Health and Medical Research Council (Canberra, ACT, Australia). Dr Andrew Udy was supported by a Clinical Research Skills Development Grant from Queensland Health (Brisbane, QLD, Australia). Dr Jason Roberts is supported, in part, by Australian Based Health Professional Research Fellowship no. 569917 from the Australian National Health and Medical Research Council.
Professor Lipman is a consultant to Astra Zeneca and Janssen-Cilag, and has received honoraria from Astra Zeneca, Janssen-Cilag and Wyeth Australia. Professor Paterson has received a research grant from Astra Zeneca and acted as a consultant for Merck, Acureon, Johnson and Johnson, Sanofi-Aventis and Three Rivers Pharmaceuticals. Astra Zeneca provides an annual non-restrictive donation to the Burns, Trauma and Critical Care Research Center (BTCCRC) at the University of Queensland (Brisbane, QLD, Australia).
The funding sources had no role in data collection, manuscript preparation, nor the decision to submit the manuscript for publication.
Author information
Authors and Affiliations
Corresponding author
Rights and permissions
About this article
Cite this article
Udy, A.A., Roberts, J.A., Boots, R.J. et al. Augmented Renal Clearance. Clin Pharmacokinet 49, 1–16 (2010). https://doi.org/10.2165/11318140-000000000-00000
Published:
Issue Date:
DOI: https://doi.org/10.2165/11318140-000000000-00000